C-GUARDIANS Pivotal Trial
Safety and Efficacy of the CGuard® Carotid Stent System in Carotid Artery Stenting (C-GUARDIANS)
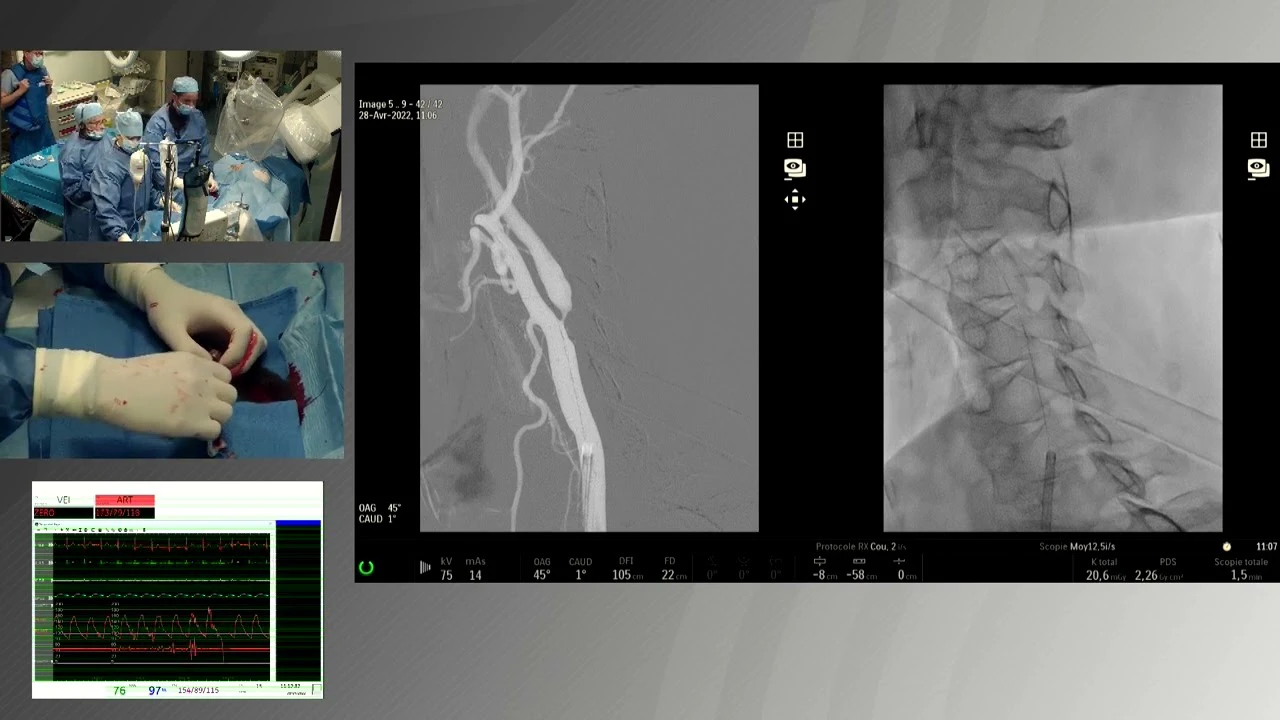
Objective: The authors sought to evaluate the safety and efficacy of the MicroNet-covered stent in treating patients with significant carotid stenosis at high risk of adverse events from carotid endarterectomy.
Conclusion: The C-GUARDIAN trial demonstrated low rates of DSMI through 30 days, and ipsilateral stroke through 1 year. No unexpected adverse device effects or unexpected serious adverse device effects were reported.