Company Completes Up-List to Nasdaq Capital Market, with Trading Becoming Effective May 21, 2021
Management to host investor conference call today, May 11, 2021, at 8:30am ET
Tel Aviv, Israel— May 11, 2021 – InspireMD, Inc. (NYSE American: NSPR), developer of the CGuard™ Embolic Prevention System (EPS) for the prevention of stroke caused by the treatment of Carotid Artery Disease (CAD), today announced financial and operating results for the first quarter ended March 31, 2021.
First Quarter 2021 and recent highlights:
- Announced the up-list to the Nasdaq Capital Market, on which the company’s stock will be traded beginning May 21, 2021
- Announced reverse stock split of 1:15 with subsequent successful up-listing of shares on the Nasdaq Capital Market.
- Announced the closing of an upsized underwritten public offering combined with other capital raising transactions in Q1 2021, which netted the Company a total of $35.1 million.
- Announced the appointment of leading interventional cardiologist Chris Metzger, M.D., as the principal investigator for planned the C-Guardian FDA trial for CGuard EPS.
- Secured China distribution agreement including investment and partnership for seeking regulatory approval for CGuard EPS in mainland China.
- Announced the engagement of Hart Clinical Consultants (HCC), a leading Contract Research Organization (CRO) to conduct the clinical trial of CGuard Carotid Stent System in the United States.
- Selected for multiple presentations for CGuard EPS, including a live demonstration during the Leipzig Interventional Congress (LINC).
“Our persistent and tireless focus on execution continues as we build on our quest to change the standard of care in the treatment of carotid artery disease and stroke prevention, with CGuard EPS and our novel MicroNet™ mesh. Our Q1 achievements have set up 2021 to be a meaningful year of progress toward our goals of global expansion, commercial revenue growth, progress toward FDA approval, growing our unmatched body of clinical evidence, and differentiating CGuard as a truly unique and preferred stent solution for carotid artery disease,” said Marvin Slosman, CEO of InspireMD.
“Most recently, we announced a successful up-listing approval of our common stock on the Nasdaq Capital Market as a part of a reverse stock split approved by stockholders. We believe listing on Nasdaq will help broaden our stockholder base, increase interest by institutional and fundamental investors, and create stockholder value. The anticipated date for our shares to begin trading on NASDAQ is May 21, 2021.
“As we ramp up the start of the C-Guardian U.S. pivotal trial for CGuard EPS — an important step in our goal to achieve commercial registration in the United States — we announced that leading interventional cardiologist Chris Metzger, M.D., system chair of clinical research at Ballard Health System in eastern Tennessee, has accepted a role as principle investigator in the U.S., along with Piotr Musialek, who will serve as co-principal investigator focusing on the European enrollment in the trial. Hart Clinical Consultants (HCC), a leading Contract Research Organization (CRO), will spearhead the effort managing the trial execution.
“Executing on our global expansion strategy, we announced this quarter an agreement with three China-based investment partners who will be responsible for conducting all necessary registration and establish distribution for the CGuard EPS in mainland China. This is a foundational building block for our overall Asia growth plan. Stroke is the leading cause of death in China, and the country is believed to be the second largest market for peripheral stent procedures. We continue our push to expand into new the markets of France, Taiwan and Korea.
“Lastly, we have strengthened our balance sheet and cash reserves and believe that we are well positioned and have the resources needed to fund our trial, global expansion and build a pipeline of new products poised to transform the access and delivery of CGuard EPS.
“Advancing into 2021, we are optimistic and encouraged by the direction of our business and the potential for CGuard EPS to change the carotid disease treatment market with the most advanced stent system available,” concluded Mr. Slosman.
Financial Results for the First Quarter ended March 31, 2021
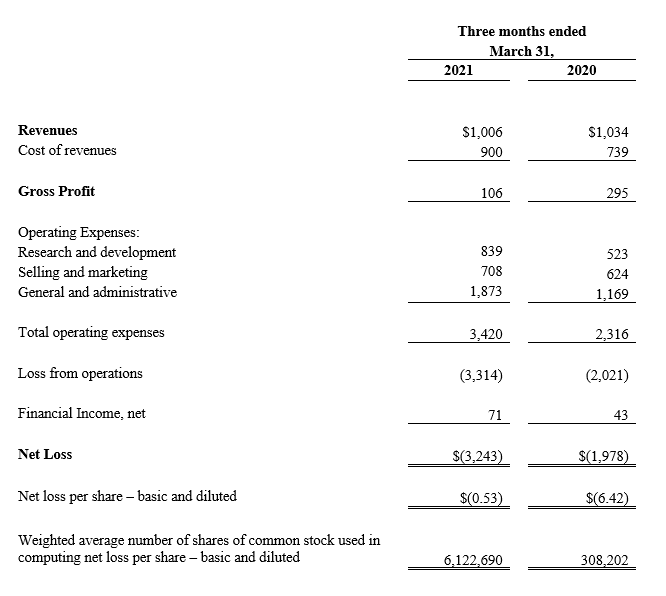
For the three months ended March 31, 2021, revenue decreased by $28,000, or 2.7%, to $1,006,000, from $1,034,000 during the three months ended March 31, 2020. CGuard revenue remained essentially unchanged at $969,000 during the three months ended March 31, 2021 as compared to $971,000 during the three months ended March 31, 2020, in spite of the continued postponement of many elective procedures as a result of the residual COVID directed resources. However, MGuard Prime EPS revenue decreased by a 41.3% from $63,000 during the three months ended March 31, 2020, to $37,000 during the three months ended March 31, 2021, largely driven by the predominant industry preferences favoring drug-eluting stents rather than bare metal stents such as MGuard Prime EPS in ST-Elevation Myocardial Infarction (“STEMI”) patients.
For the three months ended March 31, 2021, gross profit (revenue less cost of revenues) decreased by 64.1%, or $189,000, to $106,000, from $295,000 during the three months ended March 31, 2020. This decrease in gross profit resulted from an increase in write-offs of $156,000, which were driven mainly by a component supply issue during the three months ended March 31, 2021 and an increase of $33,000 in miscellaneous expenses during the three months ended March 31, 2021. Gross margin (gross profits as a percentage of revenue) decreased to 10.5% during the three months ended March 31, 2021 from 28.5% during the three months ended March 31, 2020, driven by the factors mentioned above.
Total operating expenses for the quarter ended March 31, 2021 were $3,420,000, an increase of 47.7% compared to $2,316,000 for the same period in 2020. This increase was primarily due to increases of $430,000 in salary expenses and related accrual expenses mainly driven by additional resources in our product development and sales infrastructure, $248,000 in share-based compensation-related expenses due to the expense recognition of grants made in the second half of 2020, $136,000 in development expenses associated with CGuard EPS, mainly related to the new advanced delivery system and accessories, $118,000 of Directors’ and Officers’ Liability Insurance expense due to increased premiums caused by recent trends in the overall insurance industry, an increase of $108,000 in stockholder related expenses due to the special stockholder meeting and $64,000 of miscellaneous expense.
For the three months ended March 31, 2021, financial income increased by 65.1%, or $28,000, to $71,000, from $43,000 during the three months ended March 31, 2020. The increase in financial income primarily resulted from changes in exchange rates.
Net loss for the first quarter of 2021 totaled $3,243,000, or $0.53 per basic and diluted share, compared to a net loss of $1,978,000, or $6.42 per basic and diluted share, for the same period in 2020. The average amount of shares outstanding used for the earnings per share calculation were 6,122,690 in Q1 2021 and 308,202 in Q1 2020, both adjusted to reflect the 1:15 reverse split.
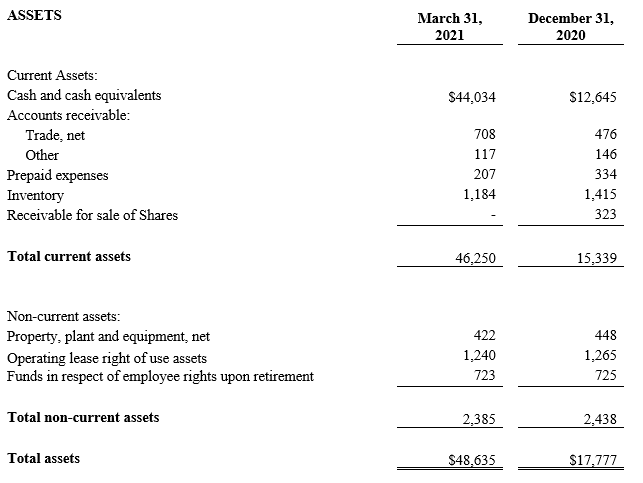
As of March 31, 2021, cash and cash equivalents were $44.0 million compared to $12.6 million as of December 31, 2020. During the first quarter of 2021, the Company raised $35.1 million net through various equity transactions.
Conference Call and Webcast Details
Management will host a conference call at 8:30AM ET today, May 11, 2021, to review financial results and provide an update on corporate developments. Following management’s formal remarks, there will be a question-and-answer session.
Please note that registered participants will receive their dial in number upon registration and will dial directly into the call without delay. Those without internet access or unable to pre-register may dial in by calling 1-844-854-4417 (domestic), 1-412-317-5739 (international) or 1-80-9212373 (Israel). All callers should dial in approximately 10 minutes prior to the scheduled start time and ask to be joined into the InspireMD call.
The conference call will also be available through a live webcast found here: https://services.choruscall.com/mediaframe/webcast.html?webcastid=yieueRnJ.
Additionally, it will be broadcast live through the Company’s website via the following link: https://www.inspiremd.com/en/investors/investor-relations/.
A webcast replay of the call will be available approximately one hour after the end of the call through August 11, 2021 at the above links. A telephonic replay of the call will be available through May 25, 2021 and may be accessed by calling 1-877-344-7529 (domestic) or 1-412-317-0088 (international) and using access code 10155884.
About InspireMD, Inc.
InspireMD seeks to utilize its proprietary MicroNet™® technology to make its products the industry standard for carotid stenting by providing outstanding acute results and durable, stroke-free, long-term outcomes.
InspireMD’s common stock is quoted on the NYSE American under the ticker symbol NSPR and certain warrants are quoted on the NYSE American under the ticker symbol NSPR.WS and NSPR.WSB.
Forward-looking Statements
This press release contains “forward-looking statements.” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company’s control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with (i) market acceptance of our existing and new products, (ii) negative clinical trial results or lengthy product delays in key markets, (iii) an inability to secure regulatory approvals for the sale of our products, (iv) intense competition in the medical device industry from much larger, multinational companies, (v) product liability claims, (vi) product malfunctions, (vii) our limited manufacturing capabilities and reliance on subcontractors for assistance, (viii) insufficient or inadequate reimbursement by governmental and other third party payers for our products, (ix) our efforts to successfully obtain and maintain intellectual property protection covering our products, which may not be successful, (x) legislative or regulatory reform of the healthcare system in both the U.S. and foreign jurisdictions, (xi) our reliance on single suppliers for certain product components, (xii) the fact that we will need to raise additional capital to meet our business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain and (xiii) the fact that we conduct business in multiple foreign jurisdictions, exposing us to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events or otherwise.
Investor Contacts:
Craig Shore
Chief Financial Officer
InspireMD, Inc.
888-776-6804
craigs@inspiremd.com
CORE IR
investor-relations@inspiremd.com
CONSOLIDATED STATEMENTS OF OPERATIONS(1)
(U.S. dollars in thousands, except per share data)
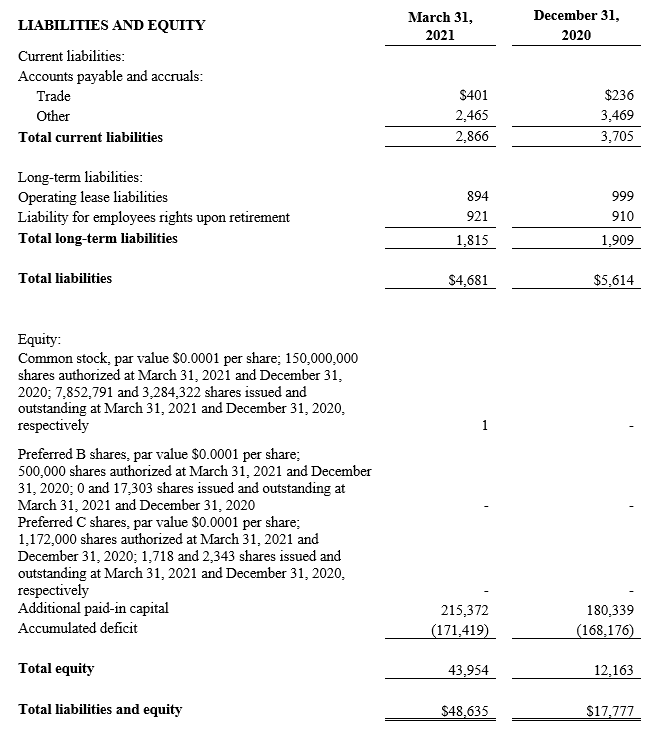
CONSOLIDATED BALANCE SHEETS(2)
(U.S. dollars in thousands)
(1) All 2021 financial information is derived from the Company’s 2021 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission; all 2020 financial information is derived from the Company’s 2020 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission.
(2) All March 31, 2021 financial information is derived from the Company’s 2021 unaudited financial statements, as disclosed in the Company’s Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission. All December 31, 2020 financial information is derived from the Company’s 2020 audited financial statements as disclosed in the Company’s Annual Report on Form 10-K, for the twelve months ended December 31, 2020 filed with the Securities and Exchange Commission.



