– CGuard™ Revenue Generated 87.5% growth over Q4’20 and 55.9% Year-over-Year –-
– Published CGuard Clinical trial results in Journals of the American College of Cardiology –
– Established Reimbursement Approval for CGuard from the French National Authority –
– CGuard Included as Carotid Stent Treatment Option in National Institute of Neurological Disorders and Stroke (NINDS) Sponsored CREST-2 Trial Following
FDA Approval of IDE Supplement Application –
—
Management to Host Investor Conference Call Today, March 8, at 8:30am ET
—
Tel Aviv, Israel— March 8, 2022 – InspireMD, Inc. (Nasdaq: NSPR), developer of the CGuard™ Embolic Prevention System (EPS) for the prevention of stroke caused by the treatment of Carotid Artery Disease, today announced financial and operating results for the fourth quarter and year ended
December 31, 2021.
Fourth Quarter 2021 and Recent Highlights:
- CGuard revenues for the fourth quarter 2021 were $1,291,000 an 87.5% increase over the same period in 2020 and for the 12-month period ended December 31, 2021, CGuard revenues totaled $4,309,000 representing a year over year increase of 55.9%.
- Appointed Katherine Arnold, to the Board of Directors and Dr. Adnan Siddiqui to the Scientific Advisory Board; each provide leadership and guidance to their respective boards with vast industry specific experience in the medical device market.
- Accelerated enrollment in the C-Guardian Investigational Device Exemption (IDE) Clinical Trial despite surging Omicron infection rates, staffing limitations and resource constraints at hospitals and healthcare systems across the United States. Under the leadership of Chris Metzger, M.D., system chair of clinical research at Ballad Health System in Eastern Tennessee the trial has 12 sites actively enrolling or recruiting patients.
- Gained approval and reimbursement coverage for CGuard EPS in France from the French National Authority for Health (HAS) and instituted a direct sales structure for commercial expansion, building on European market growth.
- Published a randomized study (SIBERIA) in Journals of the American College of Cardiology (JACC), comparing InspireMD CGuard EPS to the Abbott Acculink™, demonstrating CGuards’s 4.5-fold superiority in clinical outcomes versus this first-generation open cell Abbott stent.
Marvin Slosman, CEO of InspireMD, commented: “InspireMD continues its quest to change the standard of care for the prevention of stroke caused by carotid artery disease, through measurable progress advancing our goals of execution and growth. 2021 CGuard EPS revenue results reflected a significant rebound to the prior year with a 55.9% increase year over year, reflecting our sustained focus on commercial growth in our current 40 served markets along with continued work towards gaining approvals in both China and the United States. We advanced our pipeline of innovation with two new stent delivery accessories planned in 2022, to facilitate greater utilization of CGuard EPS as a first line stent solution for carotid artery disease management.
“In 2021, we initiated and accelerated enrollment in our ongoing C-Guardians IDE pivotal study of CGuard™ EPS (C-Guardian) in the United States, with the leadership and advisory support of renowned interventional cardiologist Gary Roubin, M.D., Ph.D., who serves on our Board of Directors, Dr. Chris Metzger, principal investigator, and lead enroller to date as well as Christina Brennan, M.D., supporting trial execution. We secured 12 of the leading interventional sites in the United States to contribute to expanding enrollment. The results to date, along with enthusiasm and feedback of the interventional community to participate and gain experience with CGuard, are an important step in maintaining our post approval commercial momentum,” Marvin Slosman added.
“In terms of our global strategy, in 2021 we continued to expand our market presence, obtaining registration and reimbursement in France along with the establishment of a direct sales presence to serve this key European market. We are currently applying for and exploring regulatory and reimbursement approvals in important new markets such as Japan and Taiwan to bolster our global footprint and advance our strategic plan for Asia.
“Our cash position remains sound, having raised an aggregate of approximately $51 million in 2020 and 2021, providing sufficient capital to continue our growth initiatives and business development plans. Our company focus and foundation is built on the superiority of our CGuard stent system, through the unique MicroNet™ mesh platform, providing sustained cerebral protection. InspireMD is committed to become an industry leader by addressing the broadest physician base of vascular specialist. We believe that expanding our leadership in transfemoral delivery of CGuard into our rapid advancing trans carotid artery revascularization (TCAR) accessory device platform will enable broader adoption of trans carotid procedural preference, thus enabling greater conversion of surgeries to stenting,” added Mr. Slosman.
“We enter 2022 with tremendous momentum, anticipating growth of CGuard, continuing pipeline development and advancing the growing body of evidence demonstrating CGuards’s superiority in clinical performance amongst all other stent options and surgery,” said Marvin Slosman, CEO of InspireMD.
Financial Results for the Fourth Quarter ended December 31, 2021
For the fourth quarter of 2021, revenue increased by $1,222,000, or 773.8%, to $1,380,000, from $158,000 during the fourth quarter of 2020. Excluding the $580,000 negative impact on revenue from our settlement of litigation with a former distributor from 2014 in the fourth quarter of 2020, total revenue increased by $642,000, or 87.0%, to $1,380,000, from $738,000 during the fourth quarter of 2020. This increase was predominately driven by an increase in revenue of CGuard EPS by $603,000, or 87.5%, to $1,291,000 for the fourth quarter of 2021, from $689,000 during the fourth quarter of 2020. This increase is mainly due to growth in market share in major markets, expansion into new markets and due to the fact that in the fourth quarter of 2021, procedures with CGuard EPS, which are generally scheduled for non-emergency procedures began to return to normal levels as compared to the fourth quarter of 2020, when procedures with CGuard EPS were postponed as hospitals shifted resources to patients affected by COVID-19 beginning in February 2020.
For the fourth quarter of 2021, gross profit increased $684,000, to $294,000, compared to a gross loss of $390,000 for the fourth quarter of 2020. This increase in gross profit resulted from the impact of the $580,000 settlement with our former distributor in 2014 which was recorded in the three months ended December 31, 2020 (as mentioned above), as well as $207,000 increase in revenues less the related material and labor costs. Gross margin increased to 21.3% during the fourth quarter of 2021 from a negative 246.8% during the fourth quarter of 2020, driven by the reasons mentioned above.
For the fourth quarter of 2021, total operating expenses were $4,225,000, an increase of $897,000, or 27.0% compared to $3,328,000 for the fourth quarter of 2020. This increase was primarily due to increases in expenses related to the commencement of the C-Guardians U.S. Food and Drug Administration (FDA) study, share-based compensation-related expenses due to the recognition of grants made since August 31, 2020 and sales and marketing expenses associated with expansion of existing and new markets.
For the fourth quarter of 2021, financial expenses decreased by 7.6%, or $10,000, to $121,000, from $131,000 during the fourth quarter of 2020. Net loss for the fourth quarter of 2021 totaled $4,097,000, or $0.53 per basic and diluted share, compared to a net loss of $3,853,000, or $1.52 per basic and diluted share, for the same period in 2020. The average amount of shares outstanding used for the earnings per share calculation were 7,796,027 in the fourth quarter of 2021 and 2,533,936 in the fourth quarter of 2020, both adjusted to reflect the 1:15 reverse split effected by us on April 26, 2021.
Financial Results for the full year ended December 31, 2021
Total revenue for the year ended December 31, 2021 were $4,495,000, an increase of 80.9% compared to $2,485,000 for the year ended 2020.
Gross profit for the year ended December 31, 2021 was $754,000, or 16.8 % of revenue, as compared to $83,000, or 3.3 % of revenue for the year ended 2020.
Total operating expenses for the year ended December 31, 2021 were $15,470,000, an increase of 47.9%, compared to $10,463,000 for the year ended 2020.
Net loss for the year ended December 31, 2021, was $14,918,000, or $2.03 per basic and diluted share, as compared to a net loss of $10,544,000, or $6.97 per basic and diluted share, for the year ended 2020. The average amount of shares outstanding used for the earnings per share calculation were 7,346,022 for the full year 2021, and 1,512,439 for the same period in 2020, both adjusted to reflect the 1:15 reverse split effected by us on April 26, 2021.
As of December 30, 2021, cash, cash equivalents and short-term bank deposits were $34.0 million compared to $12.6 million as of December 31, 2020.
Conference Call and Webcast Details
Management will host a conference call at 8:30AM ET today, March 8, 2022, to review financial results and provide an update on corporate developments. Following management’s formal remarks, there will be a question-and-answer session.
Tuesday, March 8, 2022 at 8:30 a.m. ET
Domestic: 877-407-4018
International: 201-689-8471
Conference ID: 13727214
Webcast: Webcast Link
About InspireMD, Inc.
InspireMD seeks to utilize its proprietary MicroNet™® technology to make its products the industry standard for carotid stenting by providing outstanding acute results and durable, stroke-free, long-term outcomes. InspireMD’s common stock is quoted on the Nasdaq under the ticker symbol NSPR, and certain warrants are quoted on the Nasdaq under the symbol NSPRZ.
For more information, please visit www.inspiremd.com.
Forward-looking Statements
This press release contains “forward-looking statements.” Such statements may be preceded by the words “intends,” “may,” “will,” “plans,” “expects,” “anticipates,” “projects,” “predicts,” “estimates,” “aims,” “believes,” “hopes,” “potential”, “scheduled” or similar words. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the company’s control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. For example, the company is using forward looking statements when it discusses its plans to apply for regulatory and reimbursement approvals in new jurisdictions, its belief that it has sufficient capital to continue its growth initiatives and business development plans and that it anticipates momentum in 2022, the belief that expanding its leadership in transfemoral delivery of CGuard into its rapid advancing TCAR accessory device platform, will enable broader adoption of trans carotid procedural preference, thus enabling greater conversion of surgeries to stenting and its anticipation for continuing pipeline development and advancing the growing body of evidence demonstrating CGuards’s superiority in clinical performance amongst all other stent options and surgery. Such risks and uncertainties include, without limitation, risks and uncertainties associated with (i) market acceptance of our existing and new products, (ii) negative clinical trial results or lengthy product delays in key markets, (iii) an inability to secure regulatory approvals for the sale of our products, (iv) intense competition in the medical device industry from much larger, multinational companies, (v) product liability claims, (vi) product malfunctions, (vii) our limited manufacturing capabilities and reliance on subcontractors for assistance, (viii) insufficient or inadequate reimbursement by governmental and other third party payers for our products, (ix) our efforts to successfully obtain and maintain intellectual property protection covering our products, which may not be successful, (x) legislative or regulatory reform of the healthcare system in both the U.S. and foreign jurisdictions, (xi) our reliance on single suppliers for certain product components, (xii) the fact that we will need to raise additional capital to meet our business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain and (xiii) the fact that we conduct business in multiple foreign jurisdictions, exposing us to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction. More detailed information about the Company and the risk factors that may affect the realization of forward-looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events or otherwise.
Investor Contacts:
Craig Shore
Chief Financial Officer
InspireMD, Inc.
888-776-6804
craigs@inspiremd.com
Chuck Padala, Managing Director
LifeSci Advisors
646-627-8390
chuck@lifesciadvisors.com
investor-relations@inspiremd.com
|
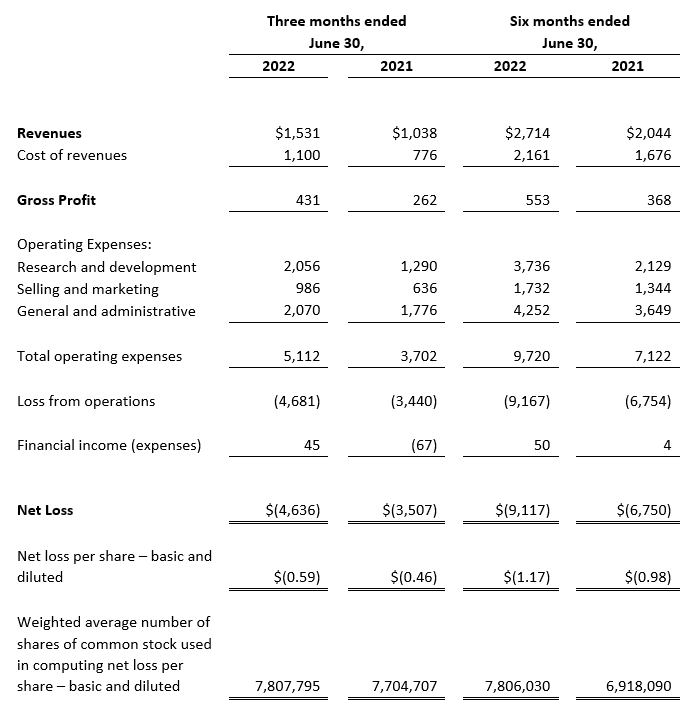
CONSOLIDATED STATEMENTS OF OPERATIONS (1)
|
|
|
(U.S. dollars in thousands, except per share data)
|
|
|
|
|
|
Twelve months ended
|
|
|
|
|
Three months ended
|
|
|
|
December 31,
|
|
December 31,
|
|
|
|
2021
|
|
2020
|
|
2021
|
|
2020
|
|
|
Revenues
|
$1,380
|
|
$158
|
|
$4,495
|
|
$2,485
|
|
|
Cost of revenues
|
1,086
|
|
548
|
|
3,741
|
|
2,402
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross Profit
|
294
|
|
(390)
|
|
754
|
|
83
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Expenses:
|
|
|
|
|
|
|
|
|
|
Research and development
|
1,534
|
|
720
|
|
5,158
|
|
2,233
|
|
|
Selling and marketing
|
761
|
|
617
|
|
2,907
|
|
2,103
|
|
|
General and administrative
|
1,930
|
|
1,991
|
|
7,405
|
|
6,127
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating expenses
|
4,225
|
|
3,328
|
|
15,470
|
|
10,463
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
(3,931)
|
|
(3,718)
|
|
(14,716)
|
|
(10,380)
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial expenses (income)
|
121
|
|
131
|
|
157
|
|
160
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before tax expenses
|
(4,052)
|
|
(3,849)
|
|
(14,873)
|
|
(10,540)
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax expenses
|
45
|
|
4
|
|
45
|
|
4
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Loss
|
$(4,097)
|
|
$(3,853)
|
|
$(14,918)
|
|
$(10,544)
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss per share – basic and diluted
|
$(0.53)
|
|
$(1.52)
|
|
$(2.03)
|
|
$(6.97)
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares of common stock used in computing net loss per share – basic and diluted
|
7,796,027
|
|
2,533,936
|
|
7,346,022
|
|
1,512,439
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
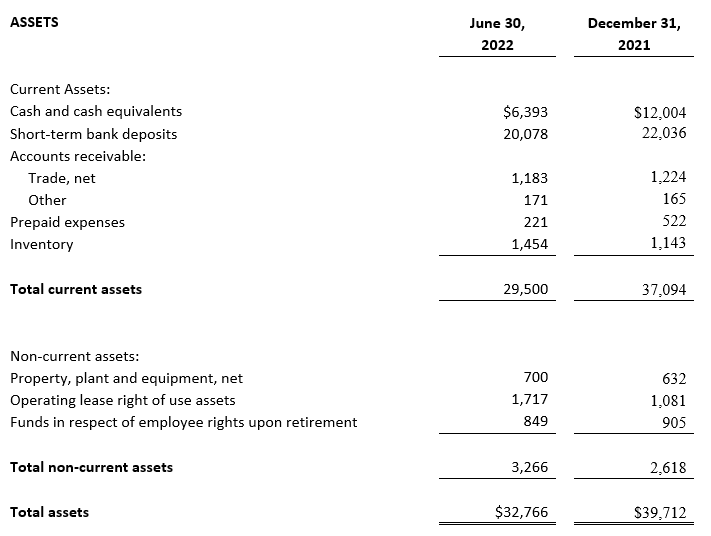
CONSOLIDATED BALANCE SHEETS (2)
|
|
(U.S. dollars in thousands)
|
|
ASSETS
|
December 31,
|
|
December 31,
|
|
2021
|
|
2020
|
|
Current Assets:
|
|
|
|
|
Cash and cash equivalents
|
$12,004
|
|
$12,645
|
|
Short-term bank deposits
|
22,036
|
|
–
|
|
Accounts receivable:
|
|
|
|
|
Trade, net
|
1,224
|
|
476
|
|
Other
|
165
|
|
146
|
|
Prepaid expenses
|
522
|
|
334
|
|
Inventory
|
1,143
|
|
1,415
|
|
Receivable for sale of Shares
|
–
|
|
323
|
|
|
|
|
|
|
Total current assets
|
37,094
|
|
15,339
|
|
|
|
|
|
|
|
|
|
|
|
Non-current assets:
|
|
|
Property, plant and equipment, net
|
632
|
|
448
|
|
Operating lease right of use assets
|
1,081
|
|
1,265
|
|
Funds in respect of employee rights upon retirement
|
905
|
|
725
|
|
|
|
|
|
|
Total non-current assets
|
2,618
|
|
2,438
|
|
|
|
|
|
|
Total assets
|
$39,712
|
|
$17,777
|
|
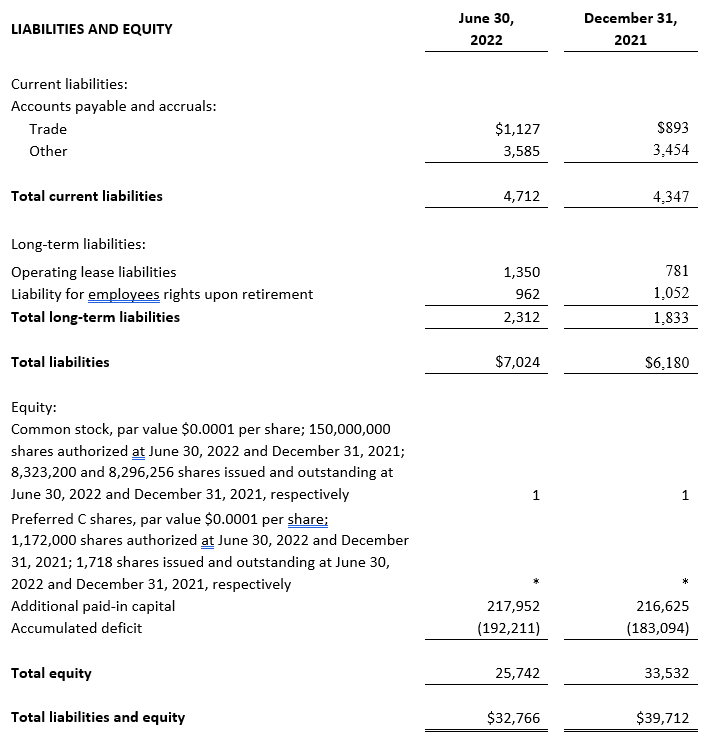
LIABILITIES AND EQUITY
|
December 31,
|
|
December 31,
|
|
2021
|
|
2020
|
|
Current liabilities:
|
|
|
|
|
Accounts payable and accruals:
|
|
|
|
|
Trade
|
$893
|
|
$236
|
|
Other
|
3,454
|
|
3,469
|
|
Total current liabilities
|
4,347
|
|
3,705
|
|
|
|
|
|
|
Long-term liabilities:
|
|
|
|
|
Operating lease liabilities
|
781
|
|
999
|
|
Liability for employee rights upon retirement
|
1,052
|
|
910
|
|
Total long-term liabilities
|
1,833
|
|
1,909
|
|
|
|
|
|
|
Total liabilities
|
6,180
|
|
5,614
|
|
|
|
|
|
|
Equity:
|
|
|
|
|
Common stock, par value $0.0001 per share; 150,000,000 shares authorized at December 31, 2021 and 2020; 8,296,256 and 3,284,322 shares issued and outstanding at December 31, 2021 and 2020, respectively
|
1
|
|
*
|
|
Preferred B shares, par value $0.0001 per share;
500,000 shares authorized at December 31, 2021 and 2020; 0 and 17,303 shares issued and outstanding at December 31, 2021 and 2020, respectively
|
–
|
|
*
|
|
Preferred C shares, par value $0.0001 per share;
1,172,000 shares authorized at December 31, 2021 and 2020; 1,718 and 2,343 shares issued and outstanding at December 31, 2021 and 2020, respectively
|
*
|
|
*
|
|
Additional paid-in capital
|
216,625
|
|
180,339
|
|
Accumulated deficit
|
(183,094)
|
|
(168,176)
|
|
|
|
|
|
|
Total equity
|
33,532
|
|
12,163
|
|
|
|
|
|
|
Total liabilities and equity
|
$39,712
|
|
$17,777
|
(1) All financial information for the twelve months ended December 31, 2021 is derived from the Company’s 2021 audited financial statements and all financial information for the twelve months ended December 31, 2020 is derived from the Company’s 2020 audited financial statements, included in the Company’s Annual Report on Form 10-K, for the twelve months ended December 31, 2021 filed with the Securities and Exchange Commission. All financial information for the three months ended December 31, 2021 and 2020 is derived from the Company’s unaudited, financial statements.
(2) All December 31, 2021 financial information is derived from the Company’s 2021 audited financial statements and all December 31, 2020 financial information is derived from the Company’s 2020 audited financial statements, as disclosed in the Company’s Annual Report on Form 10-K, for the twelve months ended December 31, 2021 filed with the Securities and Exchange Commission.