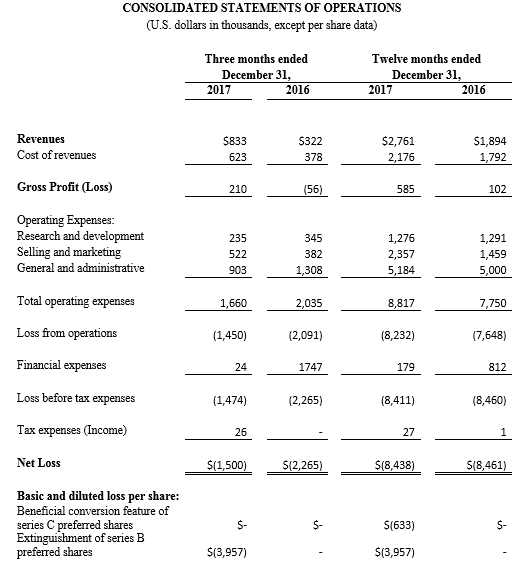
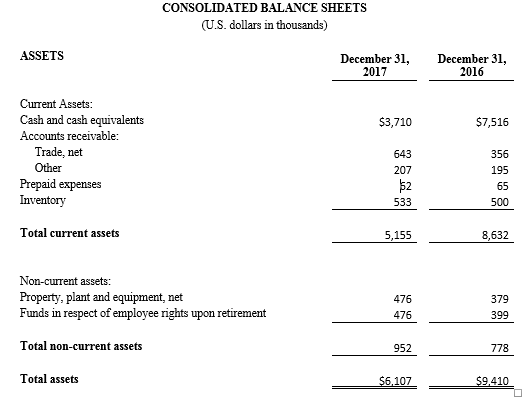
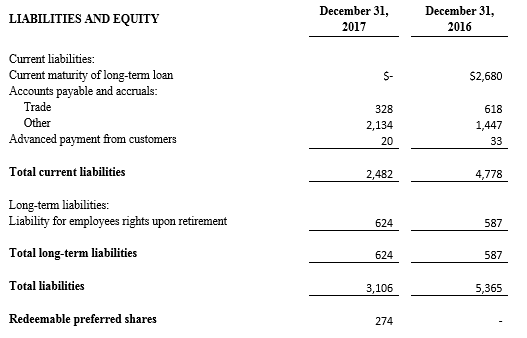
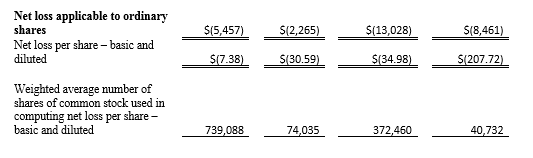Tel Aviv, Israel— February 26, 2018 – InspireMD, Inc. (NYSE American: NSPR), a leader in embolic prevention systems (EPS) / thrombus management technologies and neurovascular devices, today announced that it intends to offer and sell, subject to market and other conditions, shares of its common stock in an underwritten public offering. InspireMD also expects to grant the underwriter a 30-day option to purchase additional shares of its common stock to cover over-allotments, if any.
H.C. Wainwright & Co. is acting as the sole book-running manager for the offering. The offering is subject to market and other conditions, and there can be no assurance as to whether or when the offering may be completed, or as to the actual size or terms of the offering.
InspireMD intends to use 15% of the gross proceeds of this offering to redeem the outstanding shares of its Series D Convertible Preferred Stock, and the remainder of the net proceeds of this offering for research and development, capital expenditures, working capital and other general corporate purposes.
A shelf registration statement on Form S-3 relating to the public offering of the shares of common stock described above was filed with the Securities and Exchange Commission (“SEC”) and was declared effective on February 23, 2018. A preliminary prospectus supplement describing the terms of the offering will be filed with the SEC and will form a part of the effective registration statement. Copies of the preliminary prospectus supplement and the accompanying prospectus relating to the offering may be obtained, when available, from H.C. Wainwright & Co., LLC, 430 Park Avenue 3rd Floor, New York, NY 10022, or by calling (646) 975-6996 or by emailing placements@hcwco.com or at the SEC’s website at http://www.sec.gov. The final terms of the offering will be disclosed in a final prospectus supplement to be filed with the SEC.
This press release shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. Any offer, if at all, will be made only by means of the prospectus supplement and accompanying prospectus forming a part of the effective registration statement.
About InspireMD, Inc.
InspireMD seeks to utilize its proprietary MicroNet™ technology to make its products the industry standard for embolic protection and to provide a superior solution to the key clinical issues of current stenting in patients with a high risk of distal embolization, no reflow and major adverse cardiac events.
InspireMD intends to pursue applications of this MicroNet™ technology in coronary, carotid (CGuard™), neurovascular, and peripheral artery procedures. InspireMD’s common stock is quoted on the NYSE American under the ticker symbol NSPR and certain warrants are quoted on the NYSE American under the ticker symbol NSPR.WS.
Forward-Looking Statements
This press release includes statements relating to the proposed offering of InspireMD’s shares of common stock, including as to the consummation of this offering described above and the use of net proceeds therefrom. These statements and other statements in this press release constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company’s control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward-looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with (i) market acceptance of our existing and new products, (ii) negative clinical trial results or lengthy product delays in key markets, (iii) an inability to secure regulatory approvals for the sale of our products, (iv) intense competition in the medical device industry from much larger, multinational companies, (v) product liability claims, (vi) product malfunctions, (vii) our limited manufacturing capabilities and reliance on subcontractors for assistance, (viii) insufficient or inadequate reimbursement by governmental and other third party payers for our products, (ix) our efforts to successfully obtain and maintain intellectual property protection covering our products, which may not be successful, (x) legislative or regulatory reform of the healthcare system in both the U.S. and foreign jurisdictions, (xi) our reliance on single suppliers for certain product components, (xii) the fact that we will need to raise additional capital to meet our business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain and (xiii) the fact that we conduct business in multiple foreign jurisdictions, exposing us to foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction. More detailed information about the Company and the risk factors that may affect the realization of forward looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s Annual Report on Form 10-K and its Quarterly Reports on Form 10-Q. Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward-looking statements as a result of new information, future events or otherwise.
Investor Contacts:
InspireMD, Inc.
Craig Shore
Chief Financial Officer
Phone: 1-888-776-6804 FREE
Email: craigs@inspiremd.com
Crescendo Communications, LLC
David Waldman
Phone: (212) 671-1021
Email: NSPR@crescendo-ir.com