
Carotid Disease in 2022

So, I think I’m next, so hopefully everybody can hear me. I’m Sean Lyden, I’m the chairman of Vascular Surgery at the Cleveland Clinic.

These are my disclosures.

So, you know, I’m a surgeon, I do both TCAR, I do carotid endarterectomy, I do carotid stenting. I trained at the University of Rochester, where Charles Rob came after he had done one of the first carotids England and so he was on the wall there. So I’ve trained it an institution where it really carotid disease was developed in the United States.
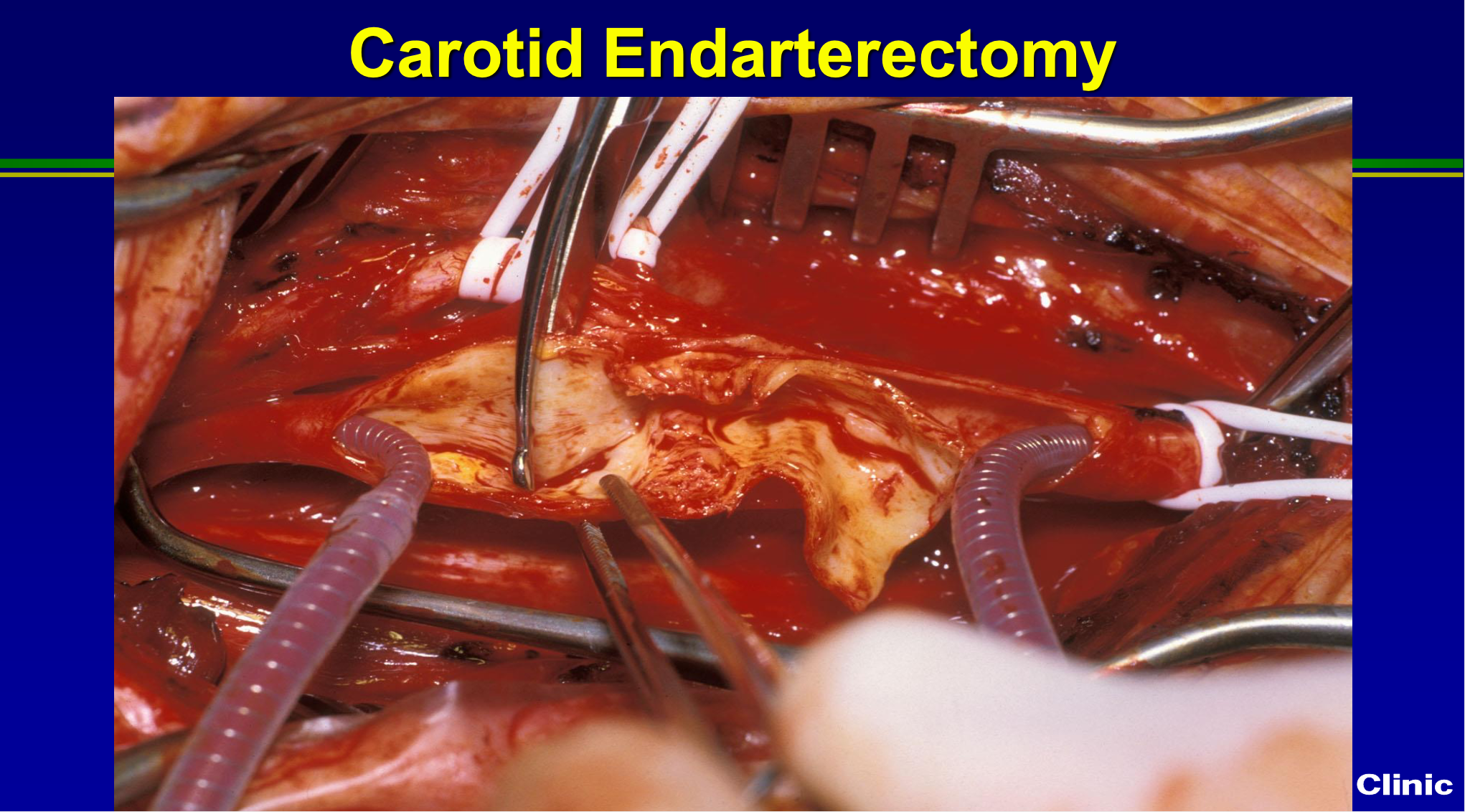
You know here’s just an operative picture, this I took from Norm Herzter, who was a chair for 15 years ago here at the Cleveland Clinic, and we still do it exactly the same way now as we did then. So we may or may not put a shunt, in we scrape all the plaque out.
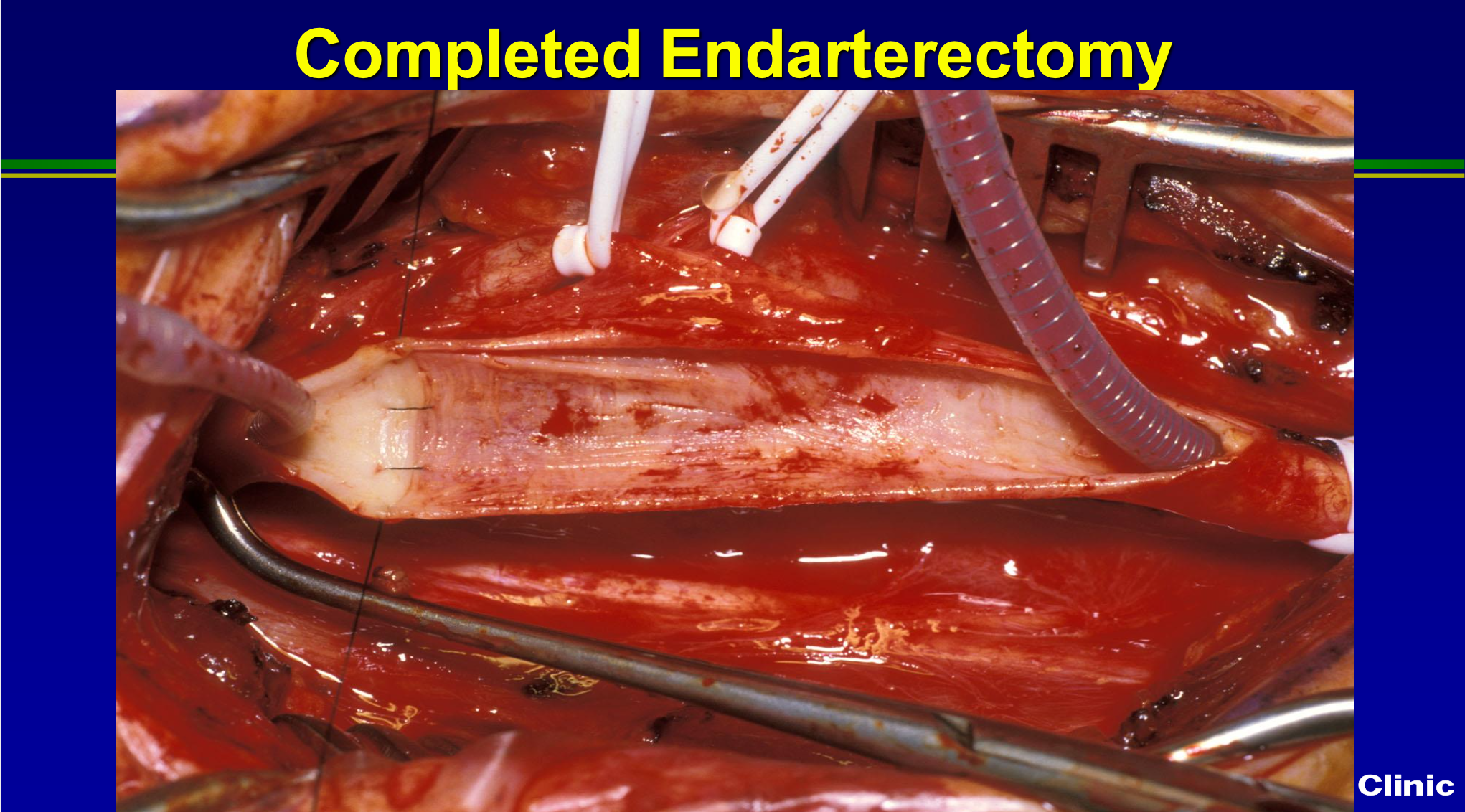
Once we’re done, we may tack down the endpoint, and see how clean it looks? Then we patch the artery to restore to normal size, or we’ll divide it and evert it, and complete it as an everted endarterectomy. But for the most part, carotid surgery is identical to the way we’ve done it for almost 30 years. We do more people under local, and we have better anesthesia techniques using narcotic based anesthesia, and so the outcomes have improved, but the surgical technical aspects are pretty much unchanged.
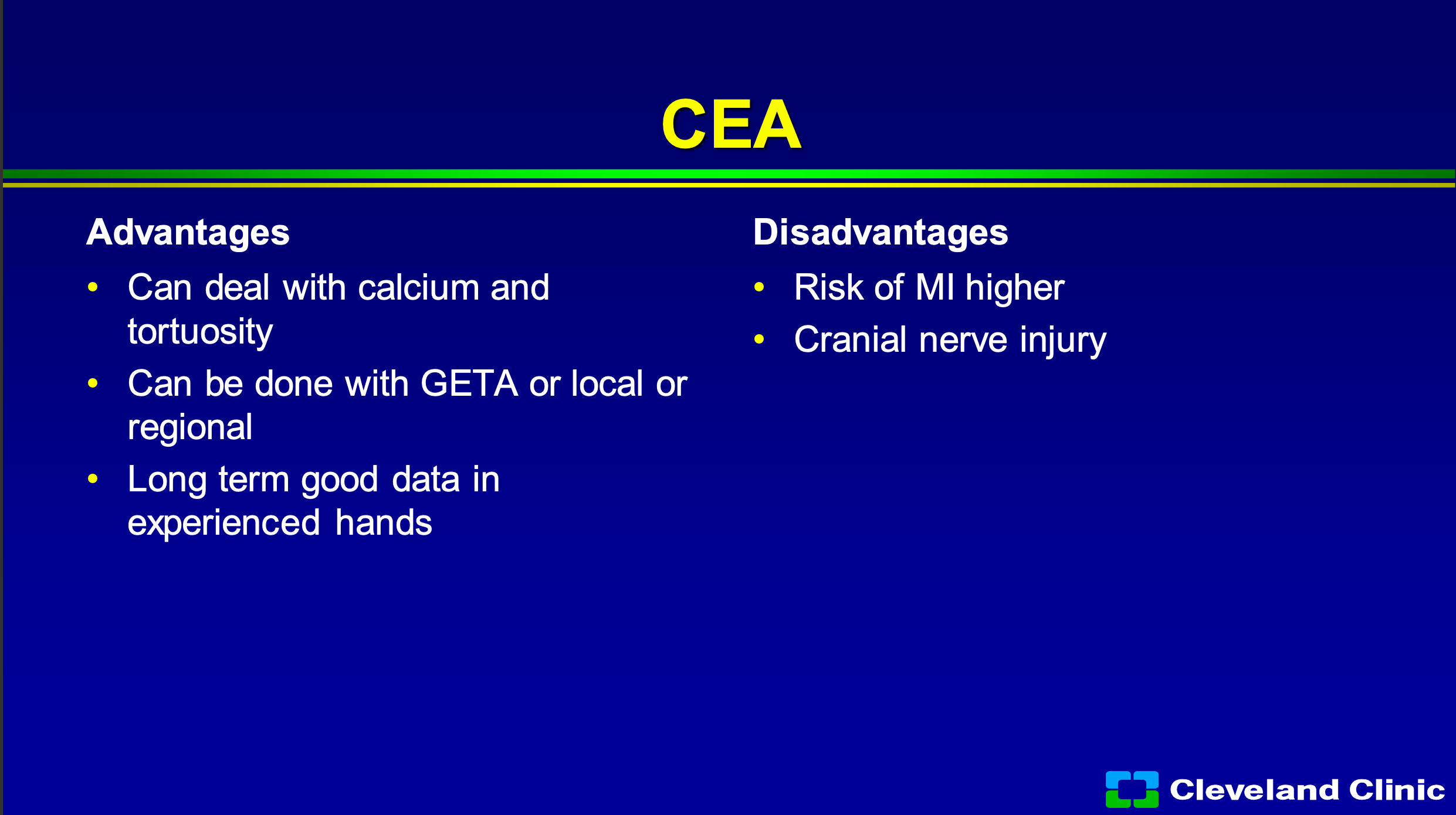
So, what are the advantages of surgery? We can deal with really dense calcium, if it’s a really tortuous arch we can deal with that. We can do people awake or asleep- when I first got to the Cleveland Clinic and we were leading in carotid stent technology, we had a summit with CEOs from everywhere and I remember I get asked if I would do a live case? Jay Yadav was doing a carotid stent on one camera, I was doing an endarterectomy on the other camera, and he goes “Now look my patient’s awake” and everybody says ‘ooh, ahh’ and I said “Hey wait look my patient’s awake too, look how awesome this is”. So you can do it either way, and it’s got great long term data. What are the downsides? Well, you know, there is definitely a higher heart attack risk, and we first saw that when we first started doing the carotid stent trials with SAFFIRE carotid stent trial, and we know patients do have cranial nerve injuries. When we look at registry studies, they say they’re 2 to 4%, when you get independent neurology evaluations, they’re definitely higher.
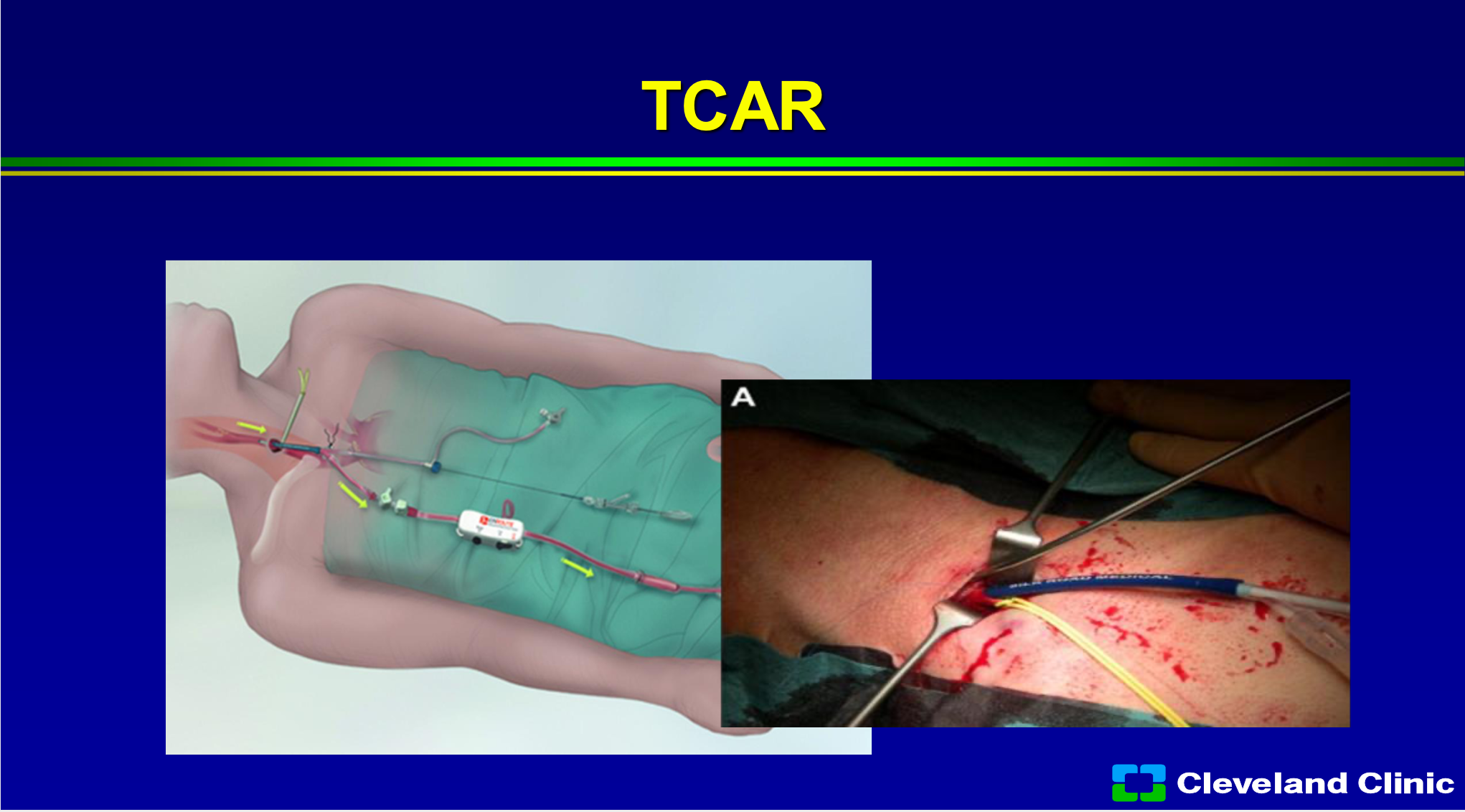
TCAR has really gained the huge interest of the vascular surgeons, and I hope none of the investors are vascular surgeons, but what I’ll sit there and tell you I think it has enabled the non- skilled interventionalist to do carotid stenting, because one of the riskier parts, when you’re learning how to do carotid stenting, is coming from the groin and getting into the carotid safely without producing a stroke. In the original carotid trials, the ACAS and NASCET trial, the angiogram alone produced about 1% of the strokes. Right now, if you’re involved in the Vascular Quality Initiative registry, you can do TCAR in patients, and so that registry is allowing the treatment of 40,000 patients in that registry, and it has grown leaps and bounds. The early results would suggest it’s about as good as surgery, however, is not a randomized study, so there are patients who you can’t treat with surgery that you can treat with TCAR, and there’s patients you can’t treat with TCAR that you can with surgery. However, there’s still cranial nerve injury, it’s still an incision, and you can cause damage to the common carotid artery. So, it is a great adjunct to what we have, but I don’t think it’s going to replace surgery in the long run, and I think most patients if they had their choice and didn’t have to have an incision in their neck still wouldn’t want it.
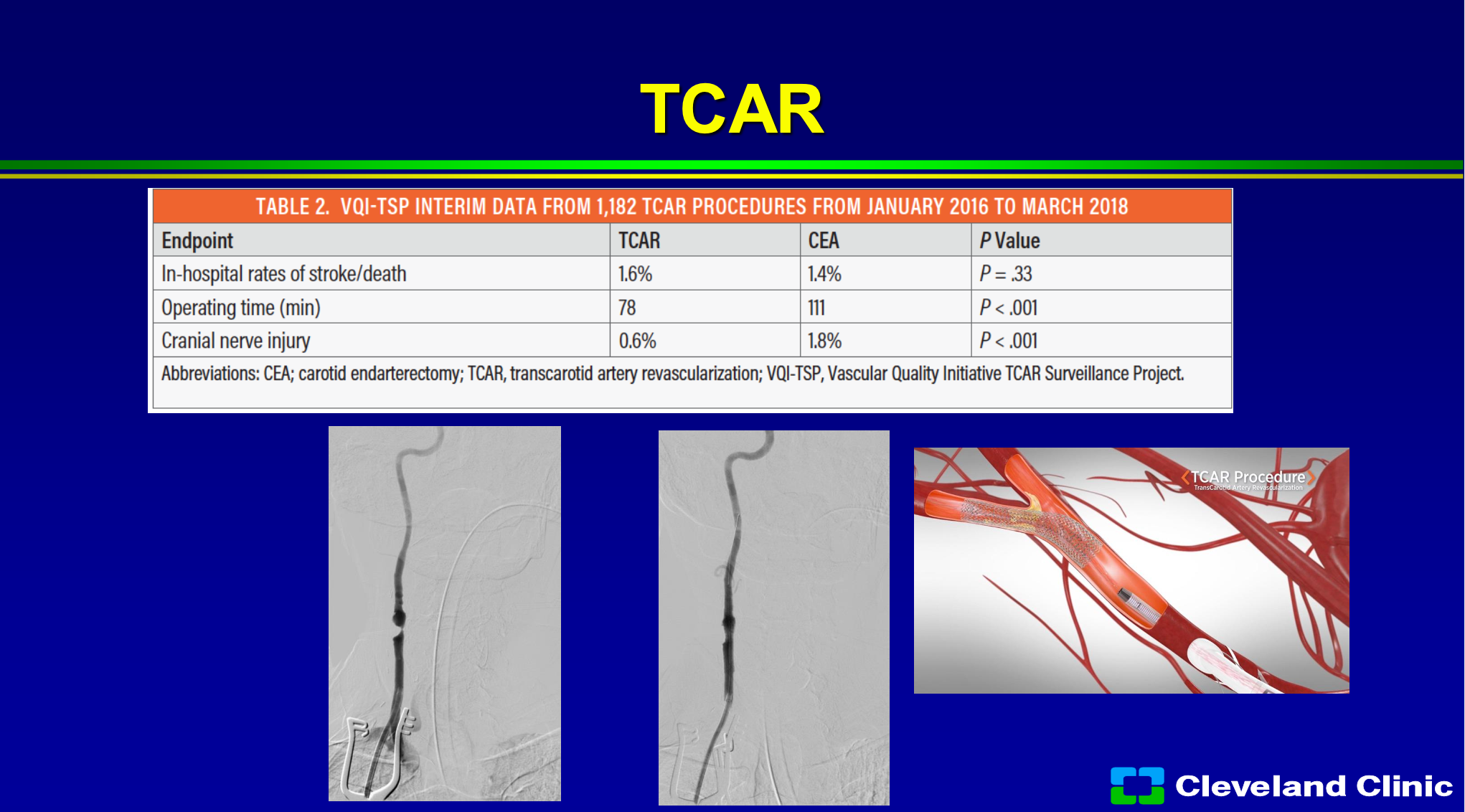
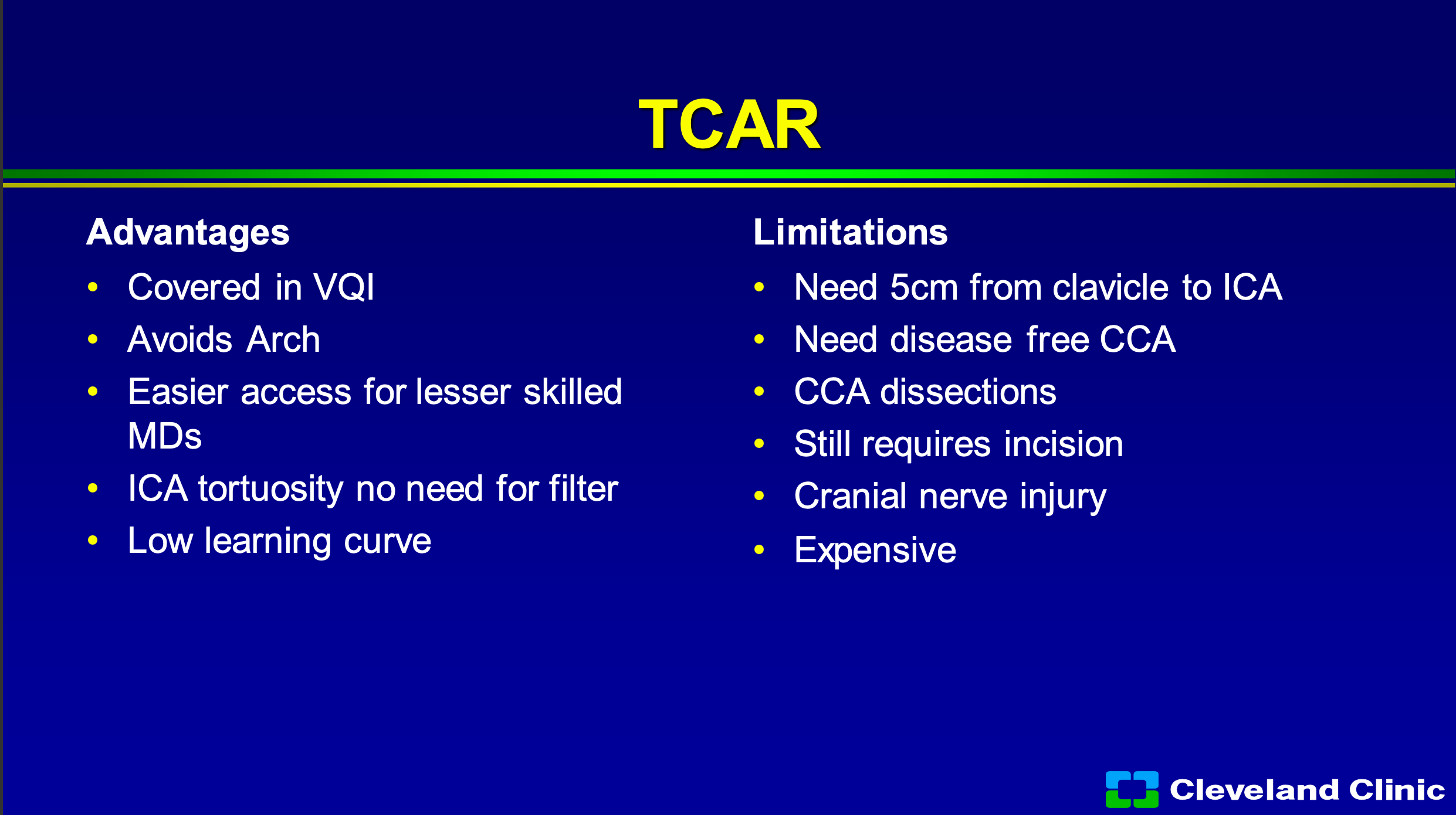
So, what are the advantages of TCAR? Well, it’s covered if you’re involved in the Vascular Quality Initiative registry, if you’re not, you still can do it. It avoids the arch, in someone for a bad arch that’s awesome. I think it clearly allows easier access for less skilled interventionalists, and if you have a really tortuous internal carotid with a loop to loop, you don’t need that distal filter. I would also say that Dr. Metzger, Dr. Siddiqui and I myself would also say that that’s where the MoMa comes in, but it has been shown to have a very low learning curve. So, unlike carotid stenting, when we first started in the early 2000s where there was a pretty steep learning curve, TCAR has been adopted pretty quickly with not a large learning curve. Some of the limitations- you need at least 5 centimeters from the clavicle to the internal carotid origin to be able to get that TCAR sheath in safely. You can’t have a big diseased common carotid artery, you can cause some dissections of the common carotid, you still need an incision, and you still have cranial nerve injury. My other job in life is I was the Medical Director of Supply Chain with the Cleveland Clinic and I’m still the chief medical officer for our GPO Accelerate, and it’s expensive, and so it costs a lot more than stenting or surgery.

Carotid stenting- we all like it, we do it and we waited forever to get it in US. The problem was in the early 2000s there was a huge fear of use and abuse and the surgeons, which I’m part of, were terrified that it was going to take over one of our last bastions of things that we only controlled. So because of the dispute between the surgical and interventional community, it was limited by Medicare to only approved trials and for approved devices and we’ve all seen how this has limited the use of the technology and the dissemination of technology.
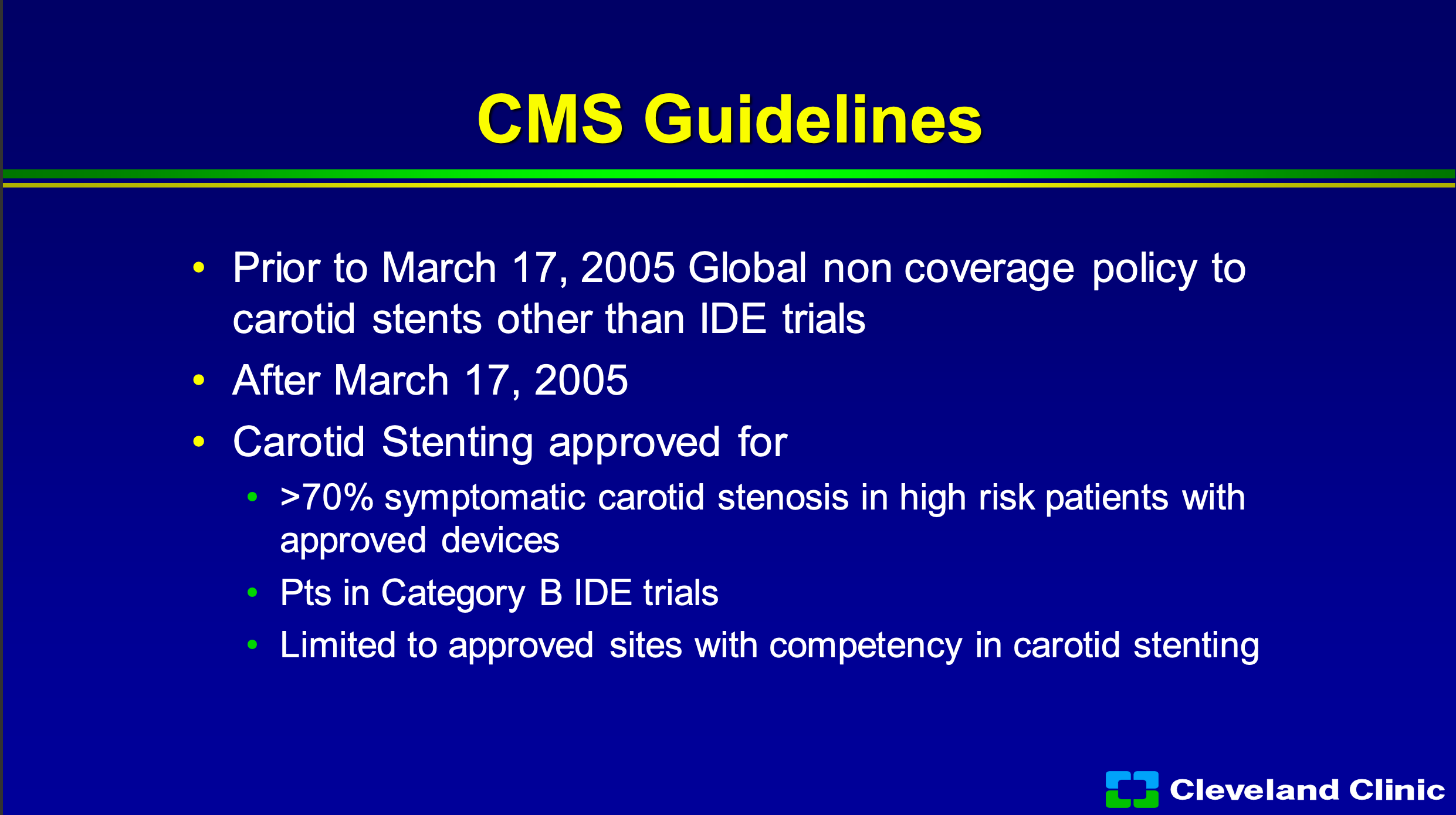
So in 2005 it was really the first time CMS, to my knowledge, really had the global non- coverage policy, so it really limited carotid stent IDE trials and it limited 70% symptomatic stenosis and high risk patients with approved devices. So you can still be in a category B trial, but you had to be a site that had to prove competency in carotid stenting and we had to report our outcomes.
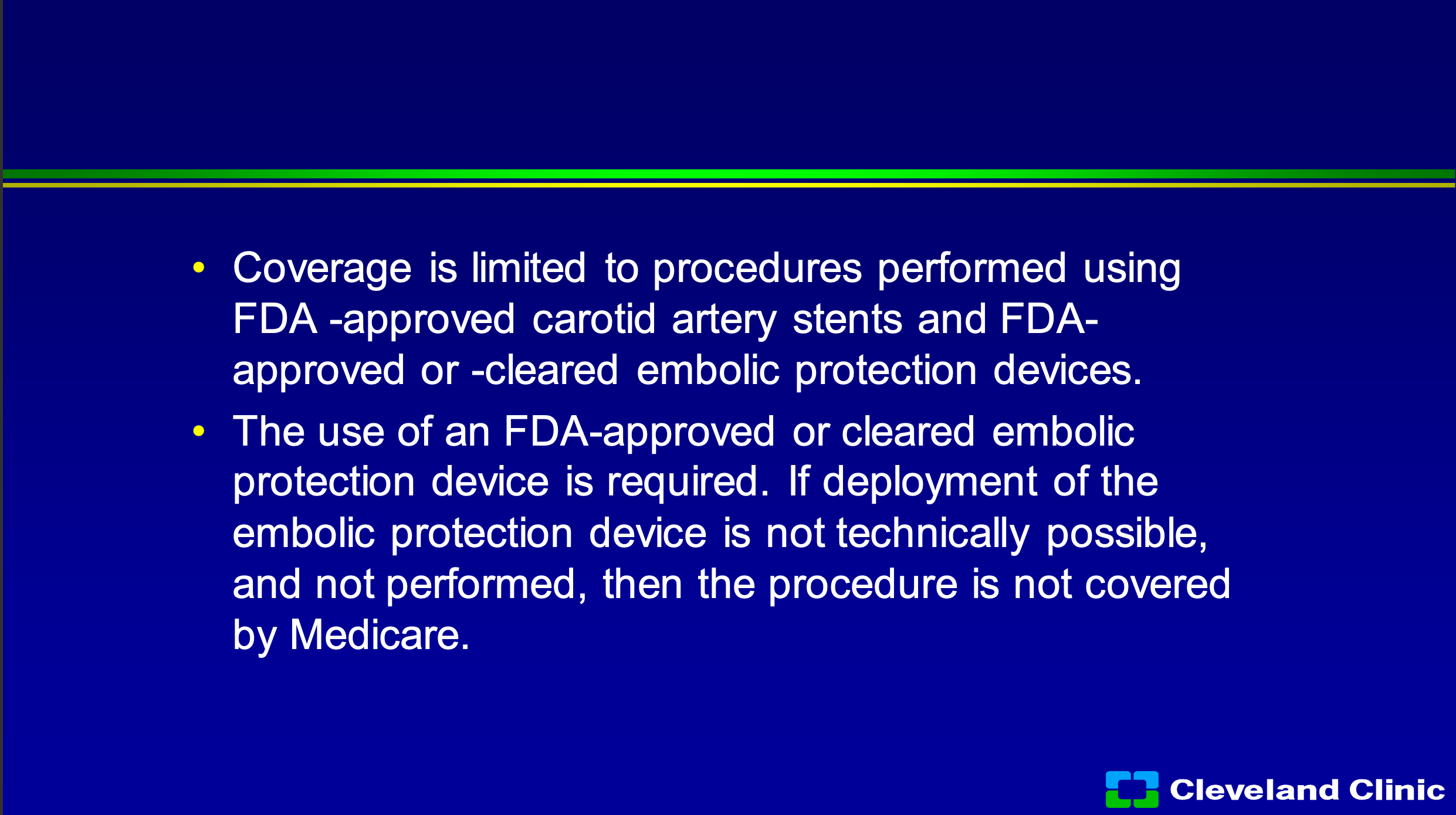
So, you can only do it with approved stents, approved devices, cleared embolic protection systems, and if you couldn’t get the embolic protection system in, CMS doesn’t pay you. Now maybe at some institutions this is not a big deal, this is a big deal to Cleveland Clinic, we’re not really excited when we do things for free, and so if patients don’t meet those coverage policies we either have to get them to sign an advanced beneficiary notice saying they’re going to pay the whole bill, or we don’t do it.
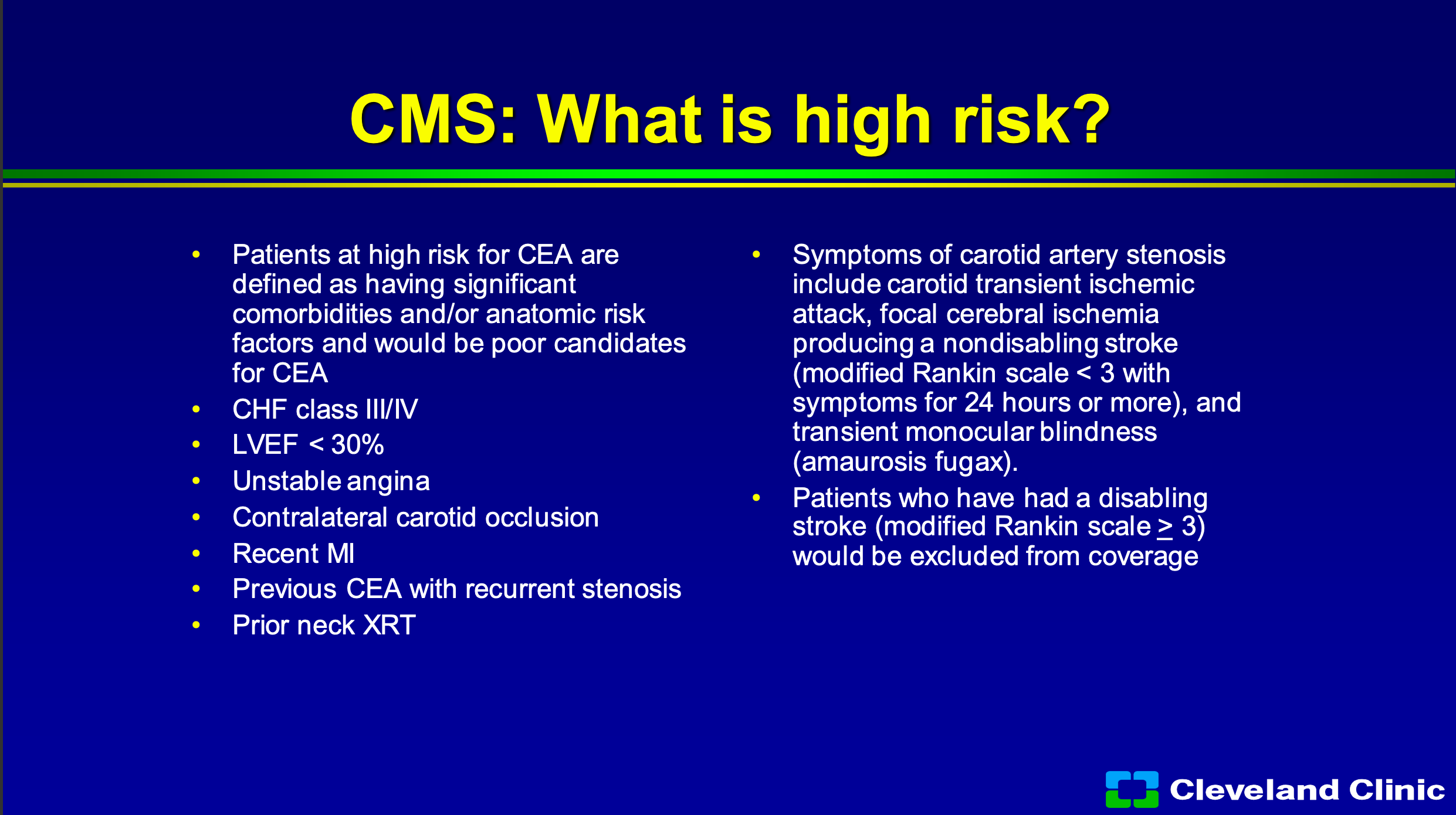
So CMS has said what’s high risk and so I’m not going to go through this, but you know you basically have to have a bad heart, bad lungs, high lesion, low lesion, or prior surgery and you have to be symptomatic with a non-disabling stroke. So that sounds like not too big a deal, right?
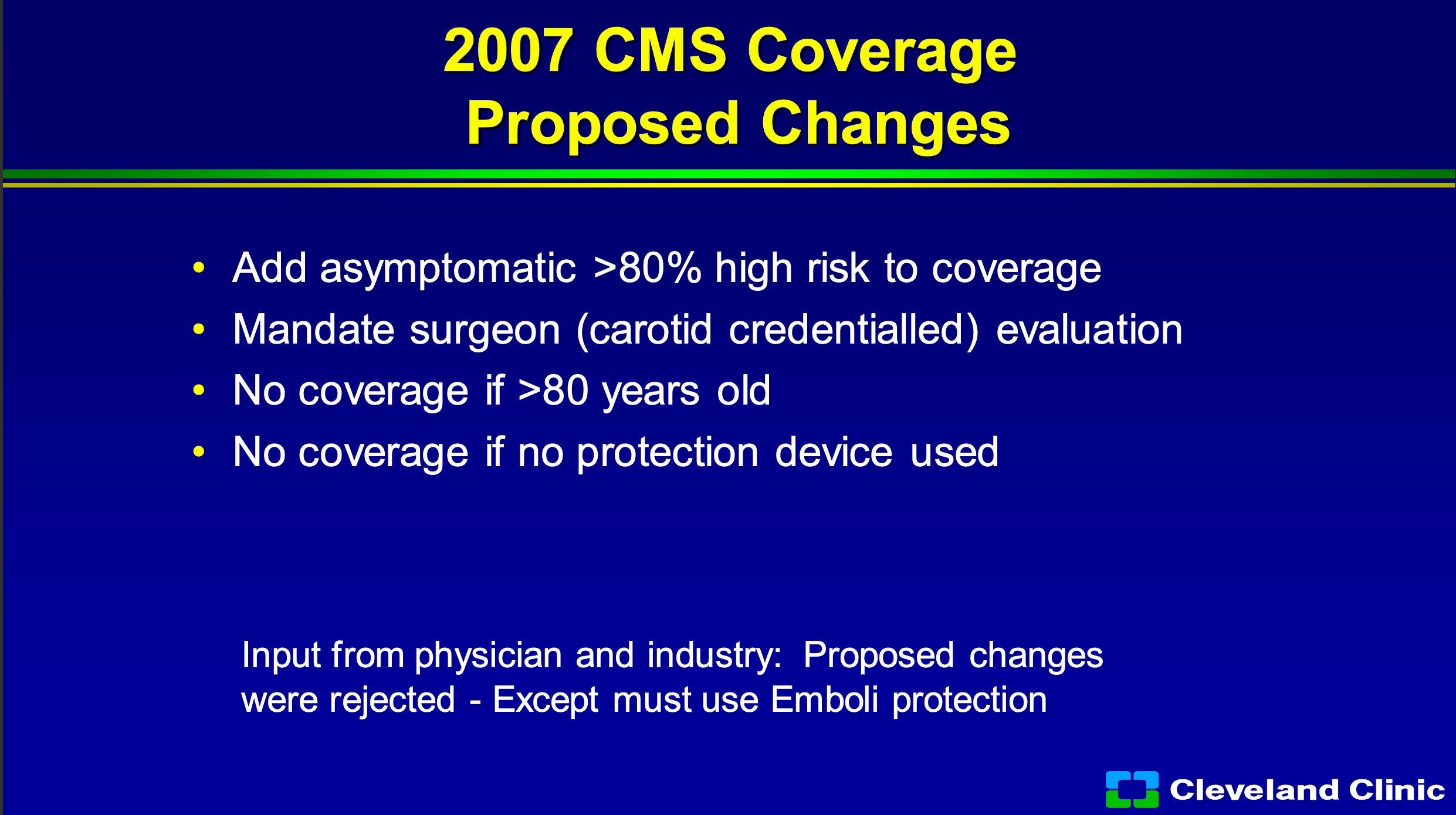
The difference is it limits to what we do, and it in my practice at the Cleveland Clinic it’s about 5 to 8% of our patients who qualify. Now, in 2007 industry and physician leaders wanted them to add asymptomatic greater than 80% to high-risk coverage. We offered mandating a surgeon evaluation and no coverage for those above 80, when the early CREST data suggested that maybe the older patients wouldn’t do as well and, no coverage of protection device used, but really CMS sort of rejected all that they basically said you still have to use embolic protection and rejected all those other suggestions.
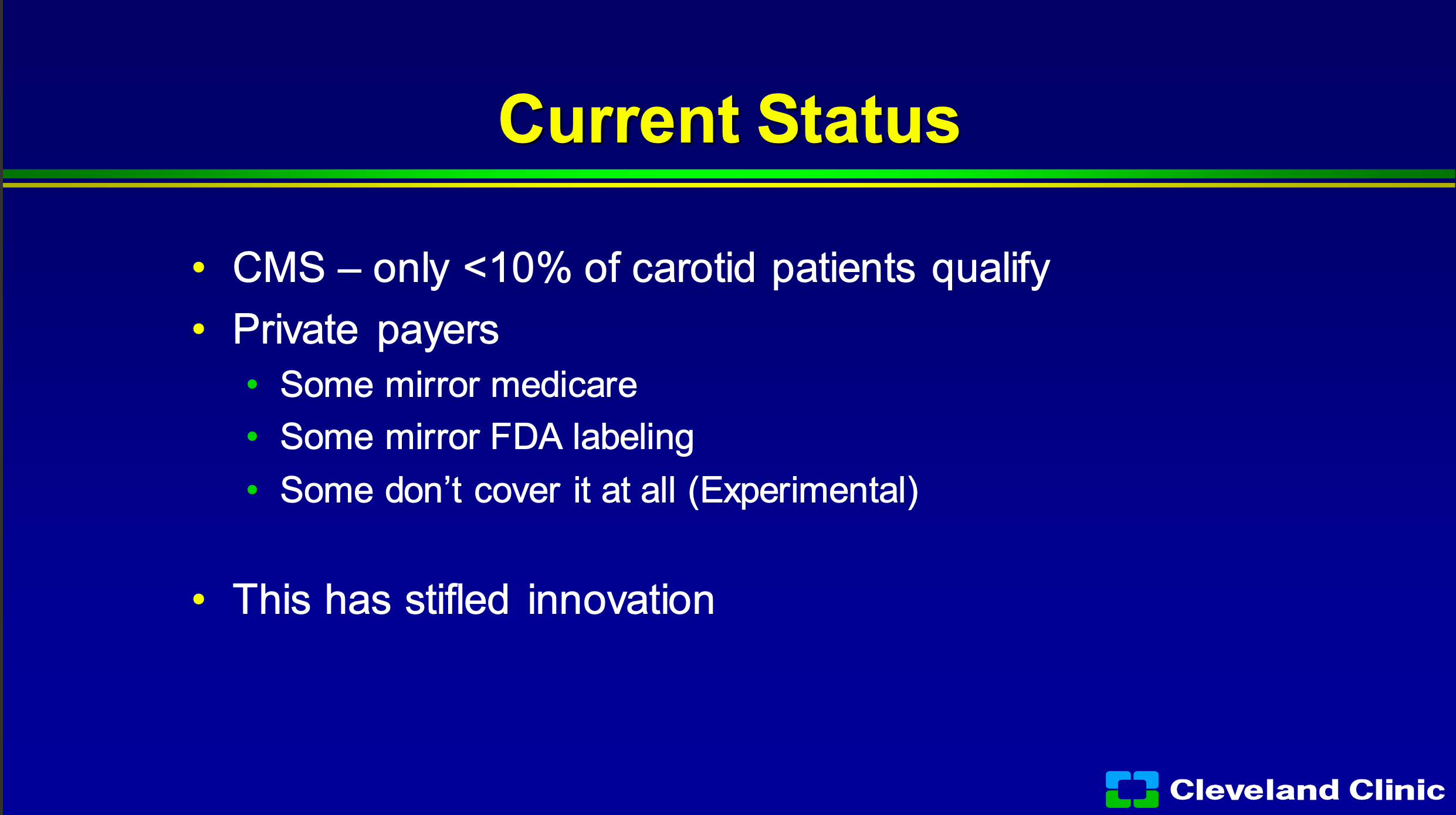
So where do we sit today in 2022? Amazingly, no different than we were in 2005. About 10% of my qualify. It’s the only disease process I do where once I decide the best way to treat my patient, I have to look down the sheet and see what they have for coverage. So if they have private carriers, some of them will mirror Medicare, some of them will mirror the FDA labeling, and some just say it’s all experimental don’t cover at all, and the reality of what this has done it has stifled innovation in this space.
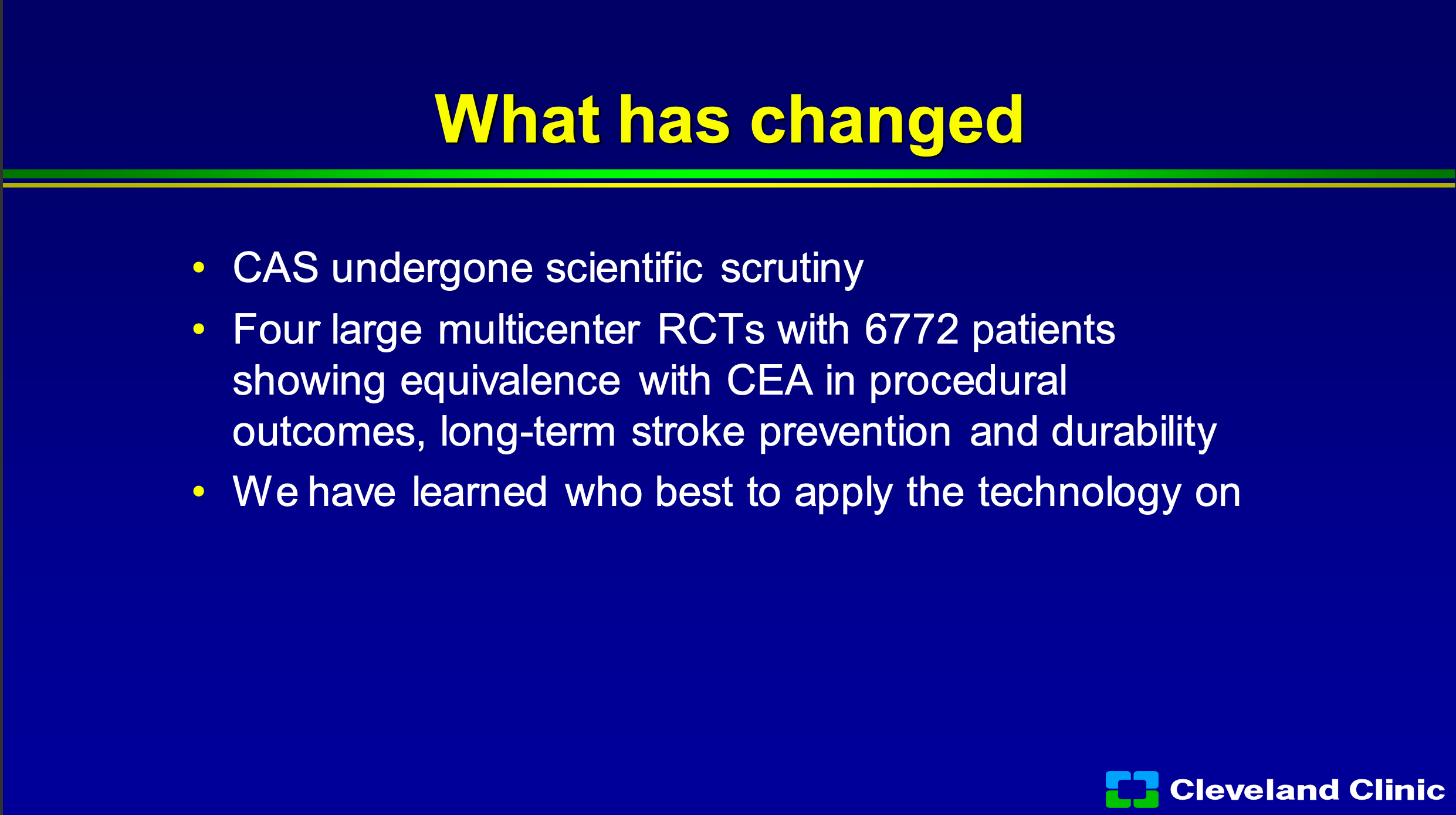
But what’s changed? Carotid stenting, despite that, has undergone the most scrutiny of pretty much any therapy I know. The differences since the last time we asked to change the coverage decision in 2007, there’s been four large multicenter randomized trials with almost 7,000 patients showing equivalence to carotid endarterectomy in both procedural outcomes, long term stroke prevention, and durability. I think we also have gotten really good as physicians in knowing who best to apply the technology on.
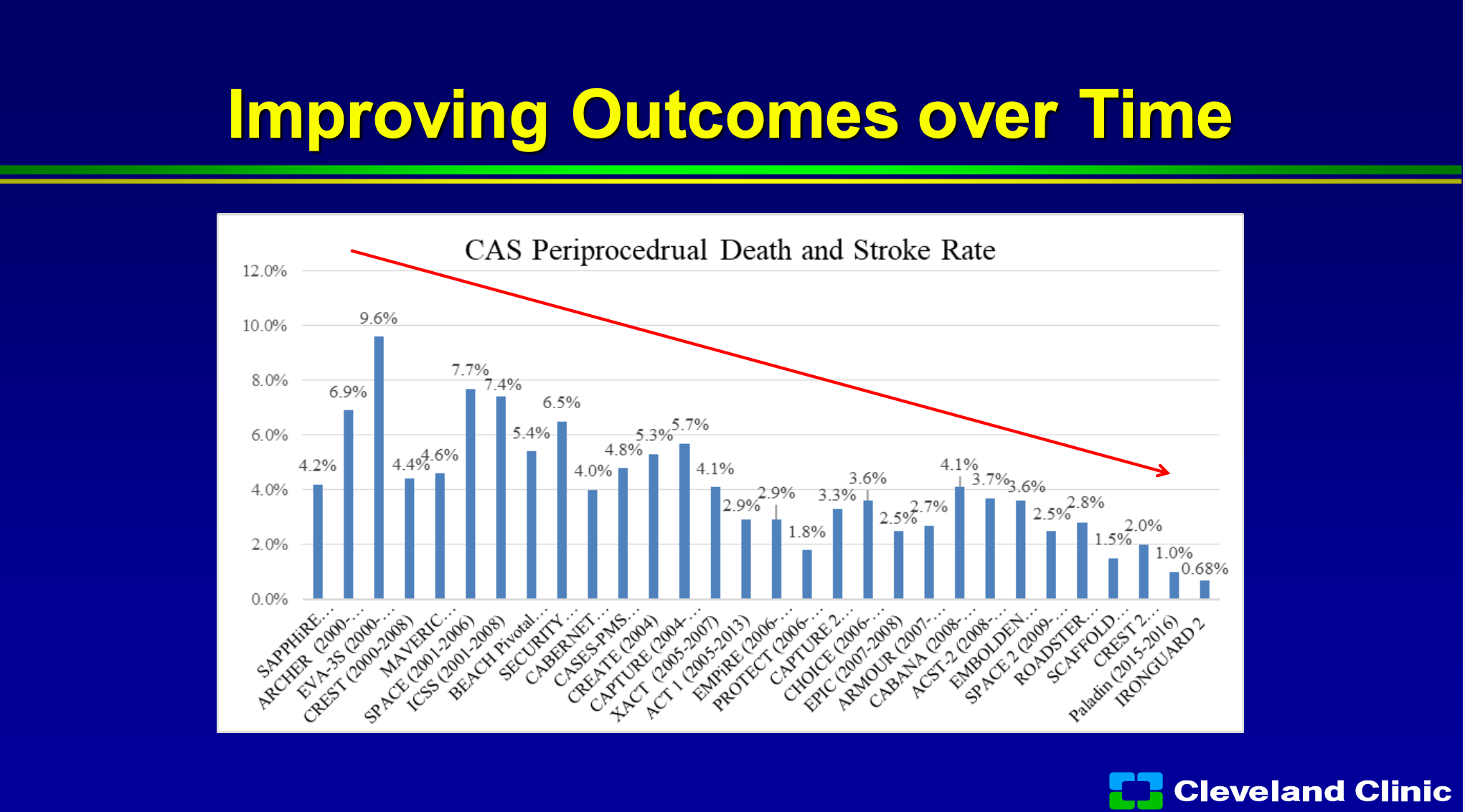
If you look at over time of all these trials, you can see the trials from the early 2000s to now, you can see that the procedural stroke and death rate has continued to improve because we’re getting better and better at figuring out on who to use this technology.
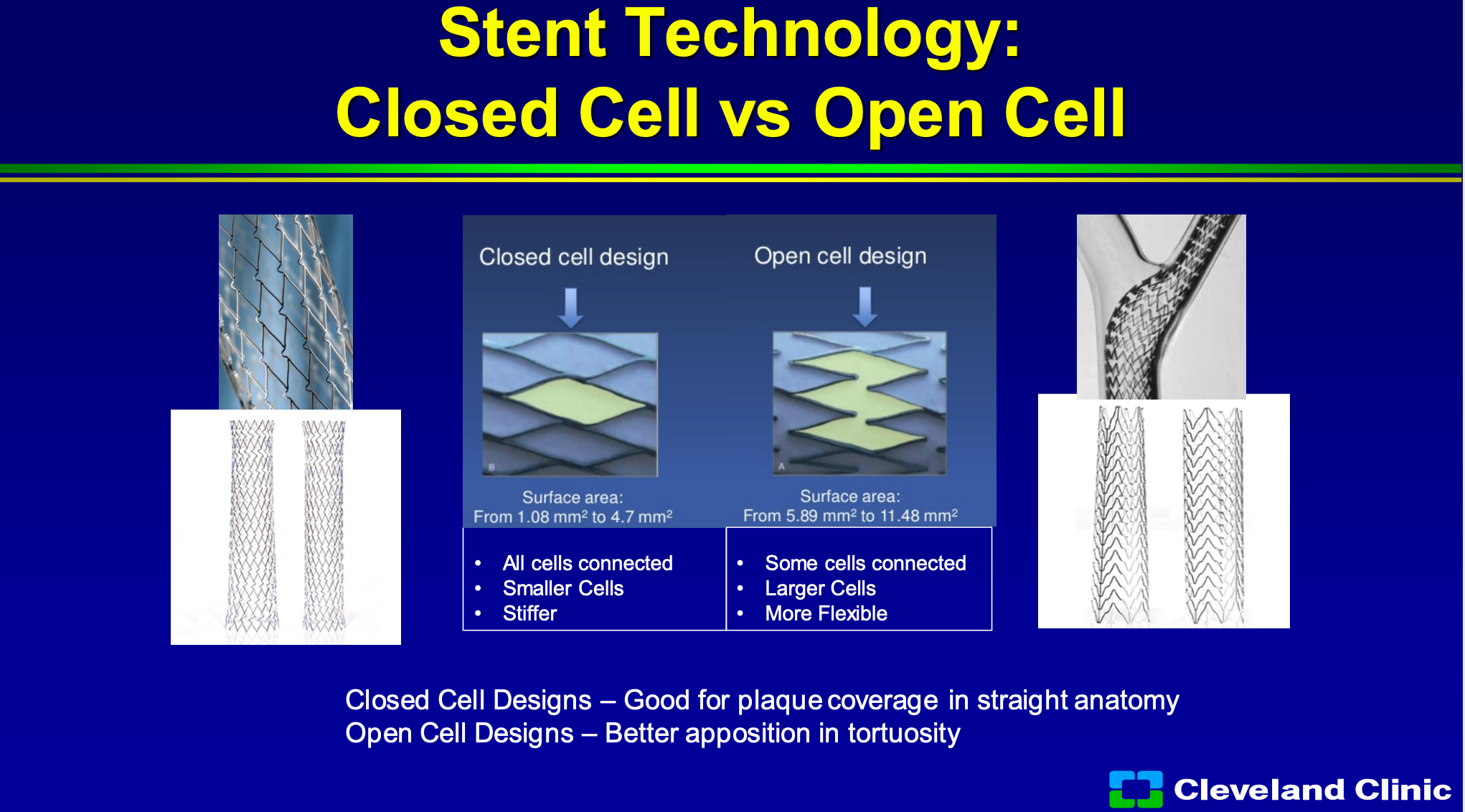
We spent forever trying to talk about closed cell and open cell, and as Chris and Adnan talked about, closed cell has better area of coverage so there’s not as much area that the plaque can come through, whereas open cell tends to have a bigger area and it allows for better conformability to the wall. So in closed cell to all the cells are connected, they’re smaller cells but it tends to be stiff, so in a torturous artery it doesn’t work well, and then in open cell design, some of the cells are connected, there are larger cells, so the ability to prolapse and they’re more flexible. So most of us have gone to say well some of the data suggest closed cell designs are better for stroke prevention and so we generally use that anytime we can or in straight anatomy, and open cell if it has torturous anatomy, and unfortunately there’s no ideal stent in there’s been this debate going on for 15 plus years.

So, you know, my opinion and many experts is that carotid stenting now should be not just for high risk patients. From the CREST outcomes, stratified by symptoms, we know that for greater than 50% symptomatic and 70% asymptomatic patients, there was no difference between carotid stenting and carotid endarterectomy for the primary endpoint of death, stroke, myocardial infarction, in either subgroup was 5.2% versus 4.5% with a hazard ratio 1.18. You can see the confidence intervals, and there was almost 1400 patients that were symptomatic and almost 1200 asymptomatic patients so a humongous trial that has reported outcomes.
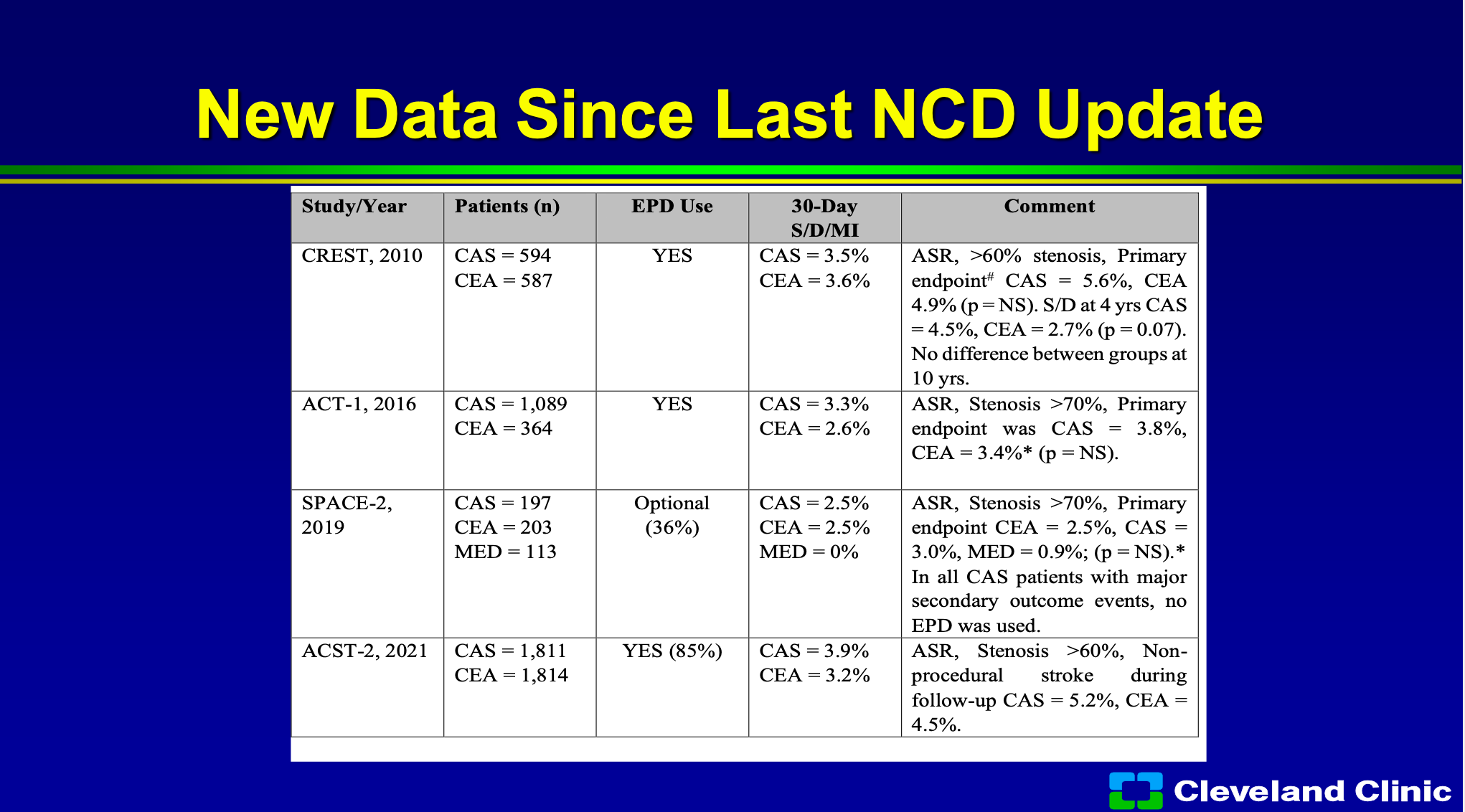
Since that last update there’s a bunch of new trials so the difference is now we have the really long term outcomes from CREST. ACT-1 one which was a randomized trial, surgery versus stenting, really, once again, showing no difference. The SPACE-2 trial carotid endarterectomy versus carotid stenting and medical management, no difference. in the ACST-2 trial just reported last year, was almost over 1800 patients in both arms was once again through late outcomes no different.
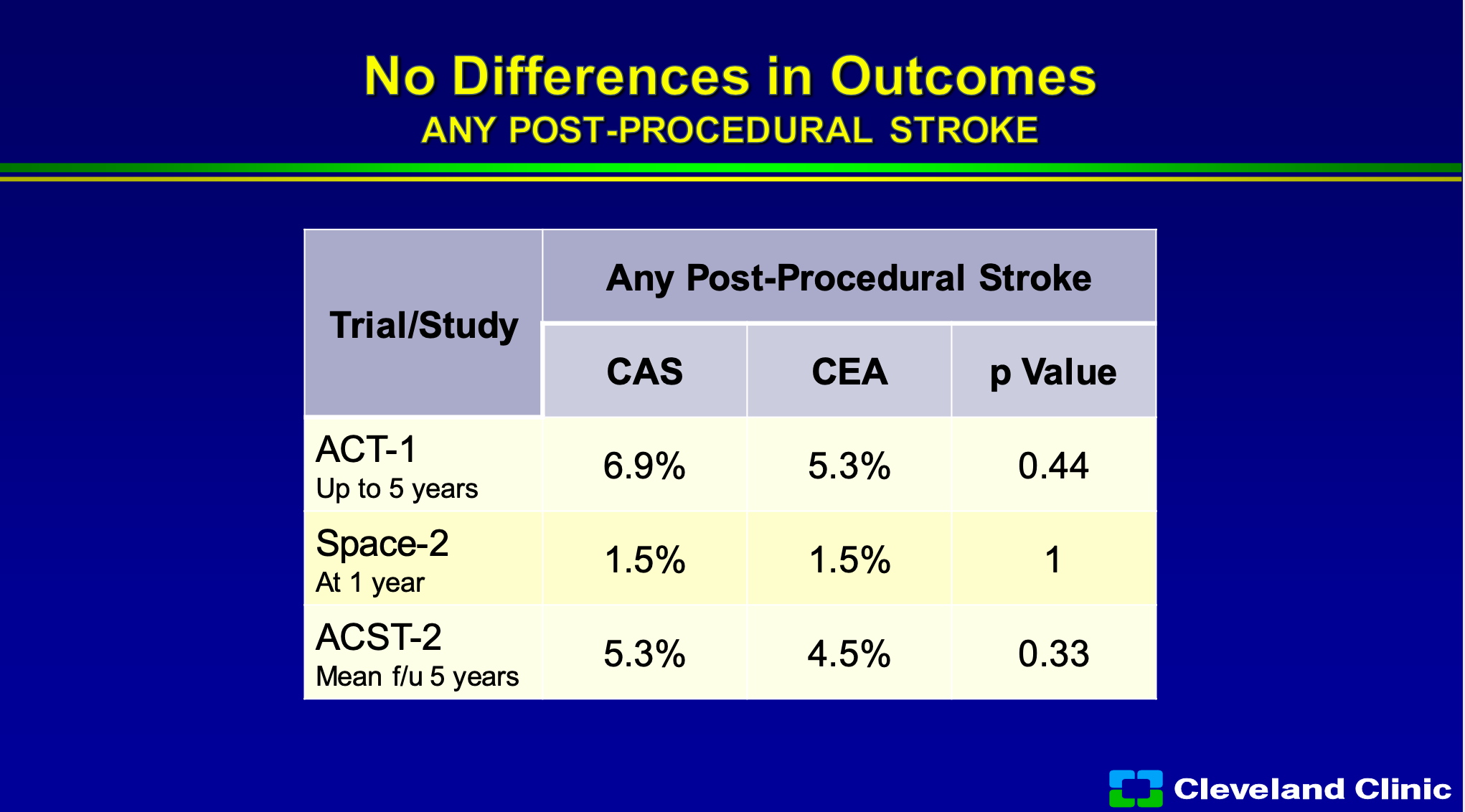
So if you look at that, we now know ACT-1 data up to five years and you can see that any procedural stroke for carotid endarterectomy, carotid stenting no difference in the P value, SPACE-2 through a year no different, ACST-2 through five years, no different.
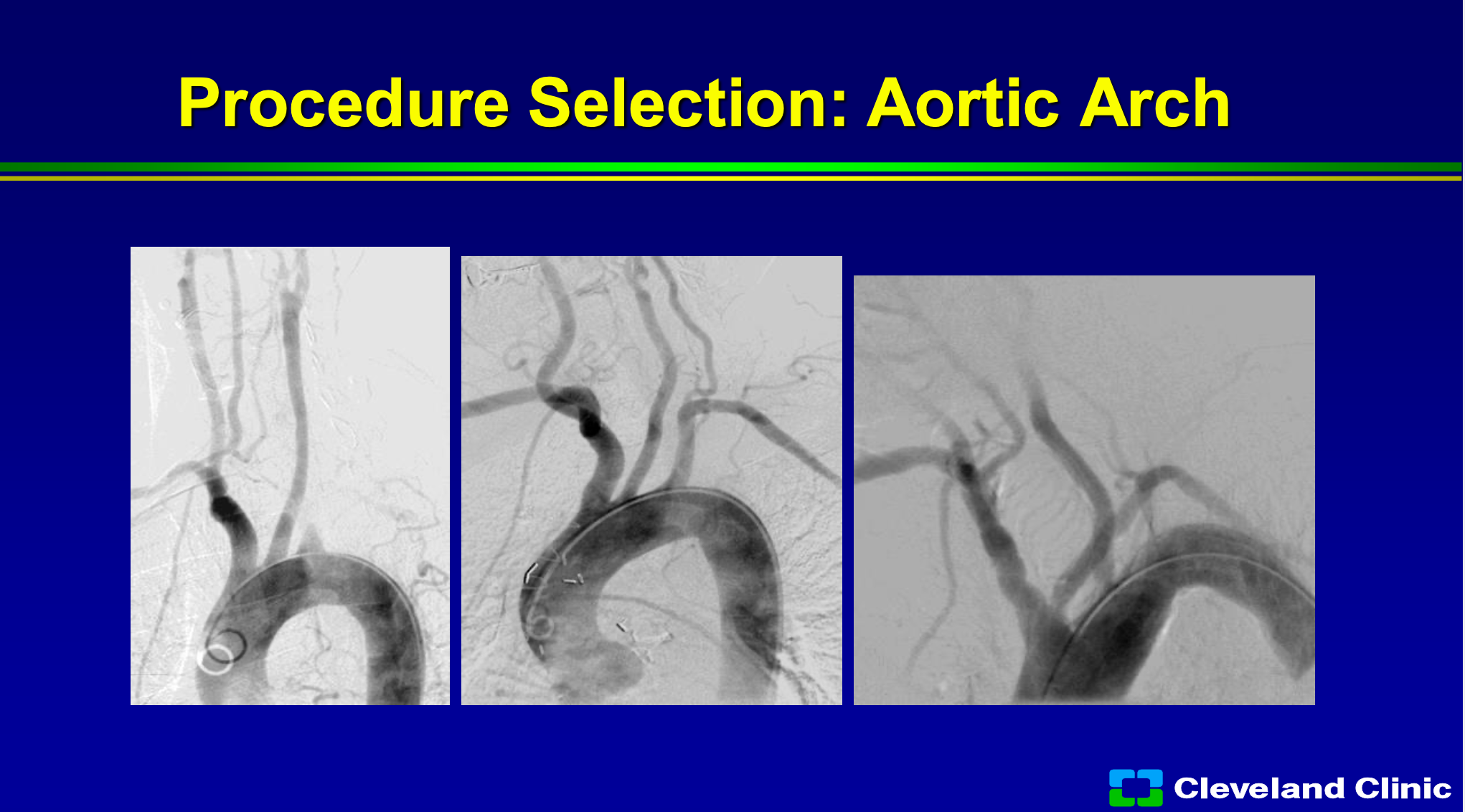
So what do we know now? We know that if we look at their arch, if it’s really that the artery comes off at the top of the arch it’s easy to get into, so the far left frame would be a type 1 arch, the far middle screen would be at more of a type 2, where the far right screen would be at type 3, so this would be one that only someone with a lot of skill might undertake or you might be able to do surgery on this patient or TCAR.
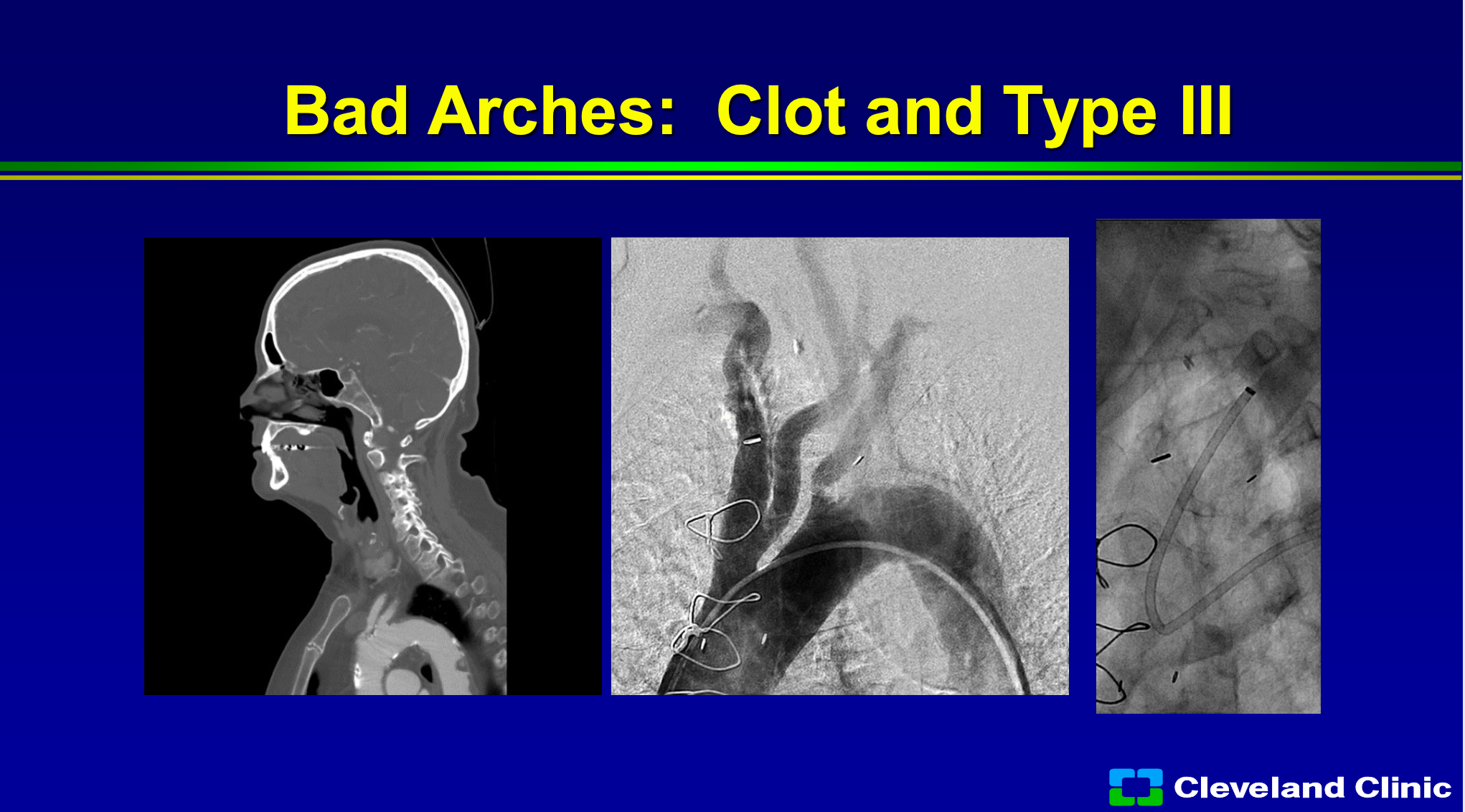
We also know that if there’s clot in the arch, or if there’s a bad arch, you can still stent people but it’s a lot harder, you’re going to have a lot more difficult risk. You can see in this patient that the sheath has kinked and now the delivery of any of your protection devices can be much more difficult with a higher risk.
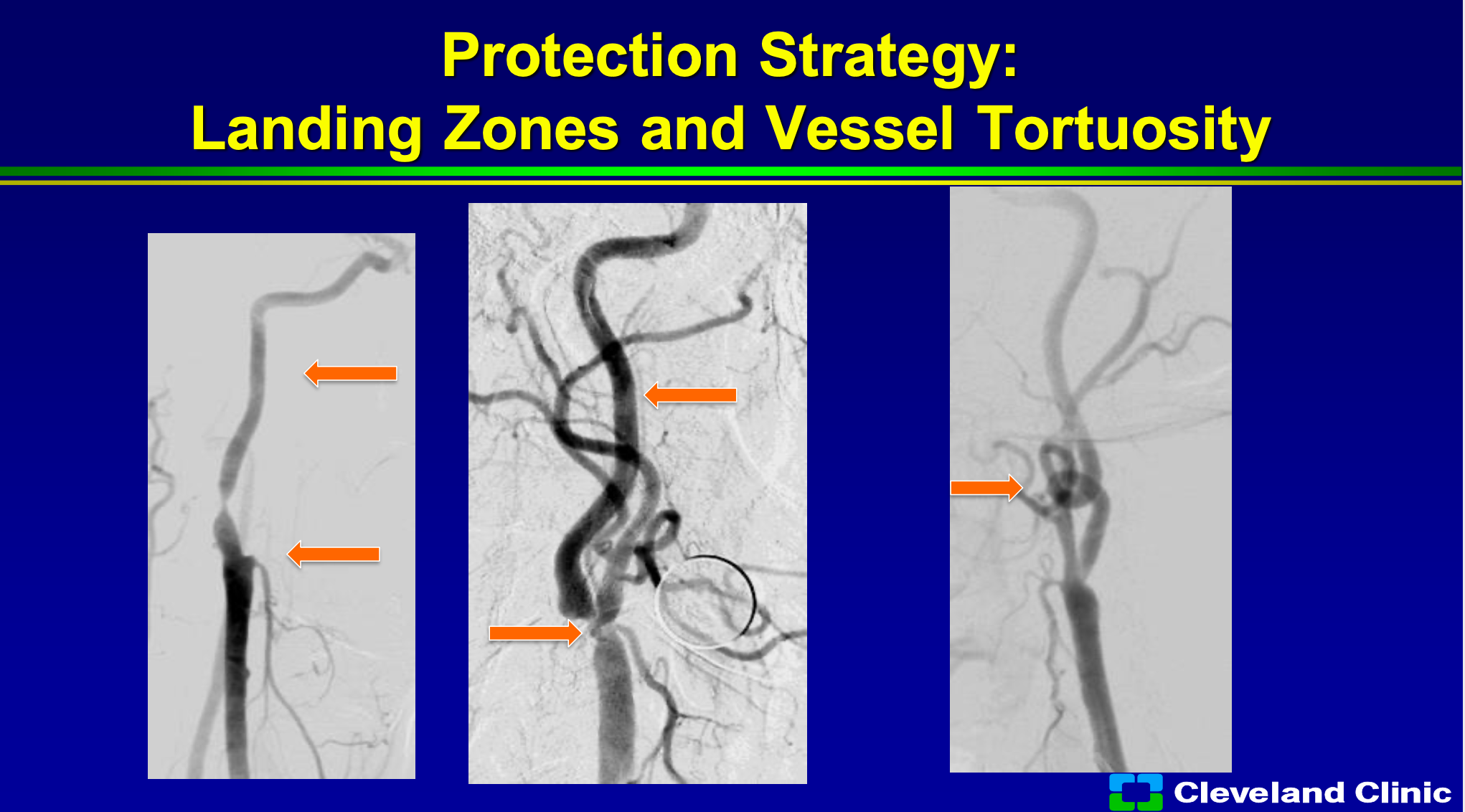
Everything we know is that where are your landing zones? So you can see patient here where the external carotid is out, so then using the MoMA or trying to get your sheath in is a little bit more difficult but not undoable. If the patient has a very straight landing zone that’s also great, but if there’s a loop to loop or tortuosity then trying to place your filter in that, it’s going be more difficult with more fraught complications.
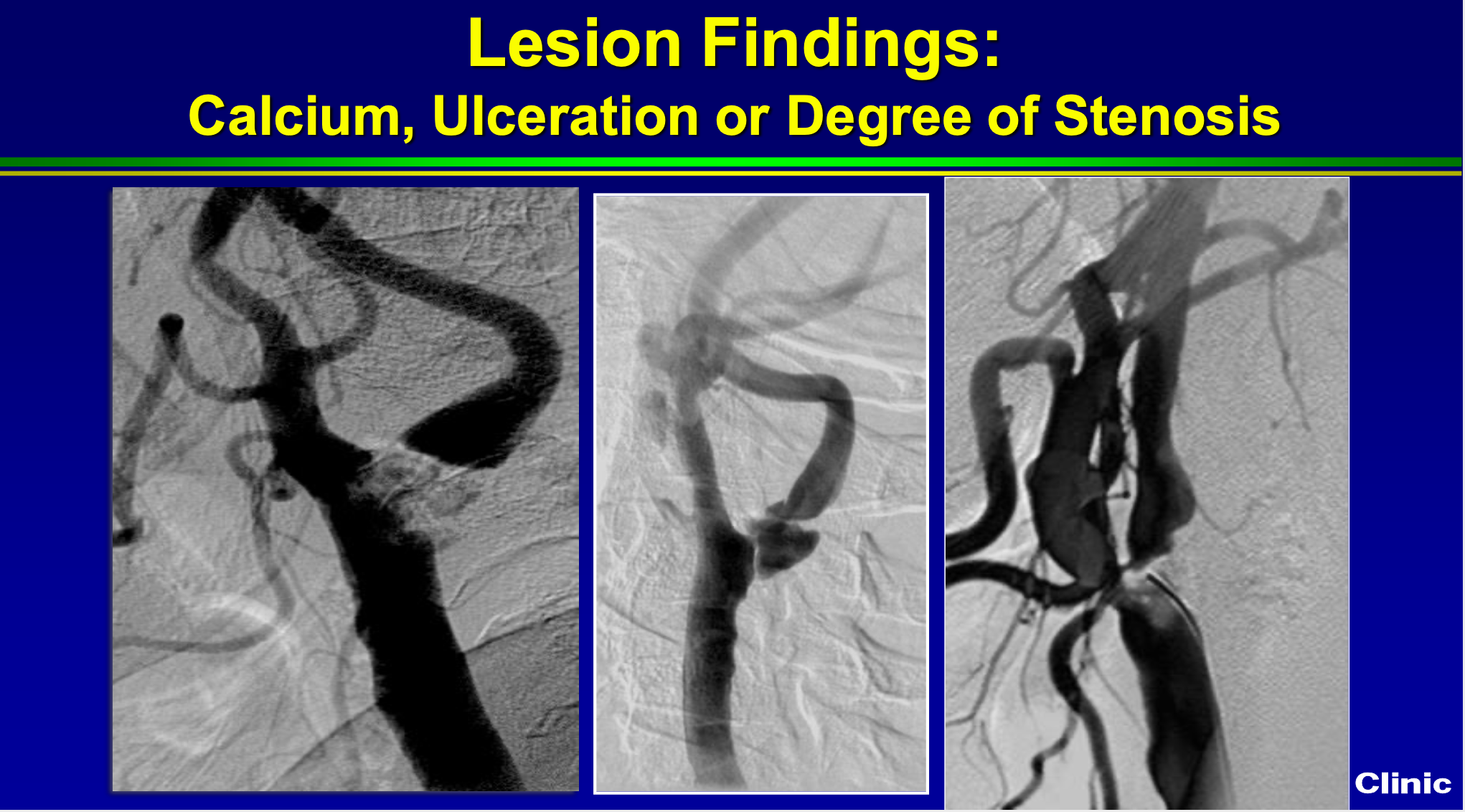
We also know that if it’s densely calcified like this picture on the left, if it has ulcerations like the middle picture, or if it’s really really tight like the picture on the right that pretends to a higher risk of adverse events with carotid standing. In skilled hands still can be done safely, but higher risk.
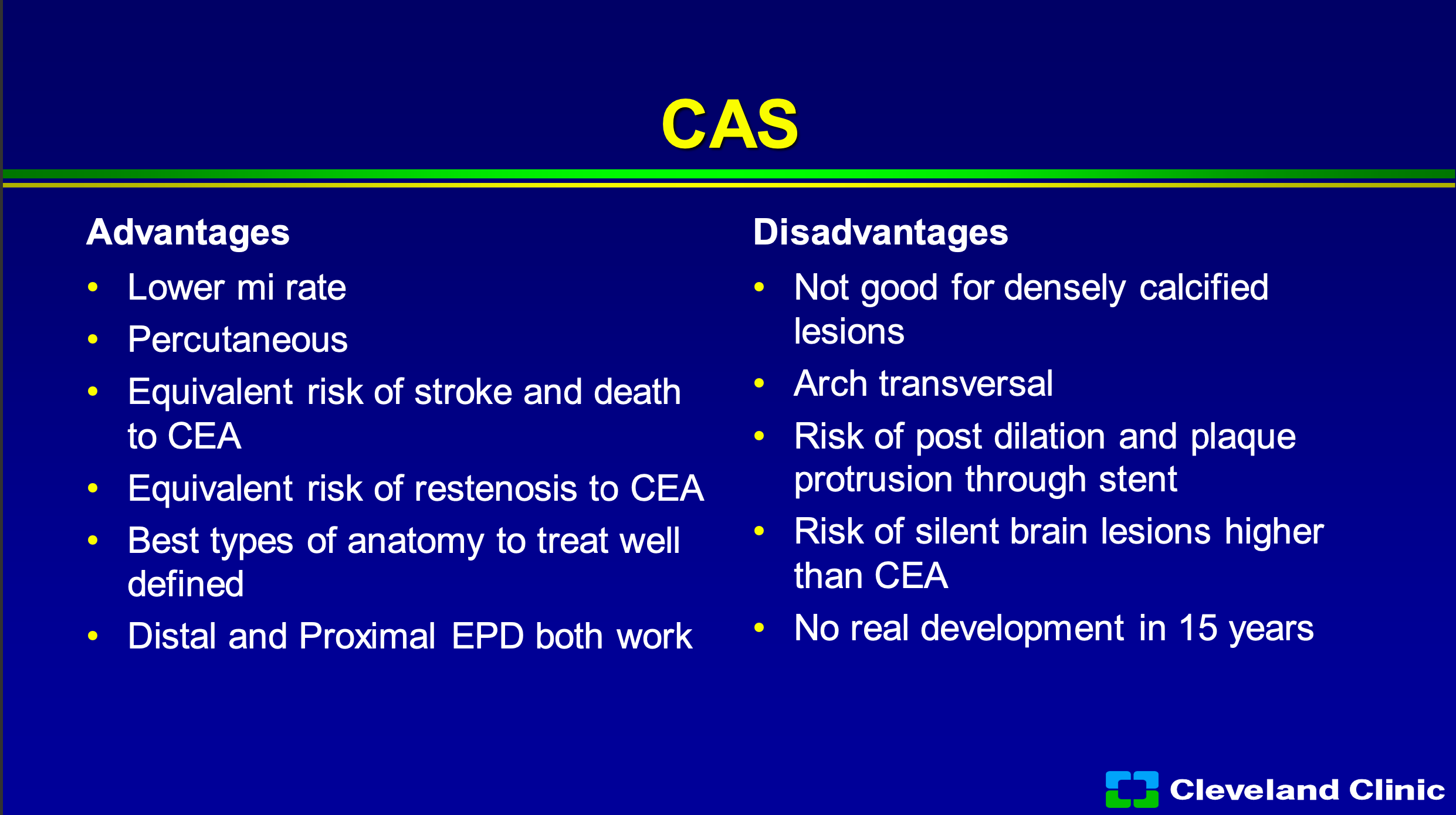
So what’s the advantage of carotid stenting? Definitely is a lower MI rate. You know I’m a surgeon, but it doesn’t take anybody to convince the patient, no scar or a scar, percutaneous is better. It has equivalent risk of stroke and death to carotid endarterectomy, it has an equivalent risk of restenosis to carotid endarterectomy, and the best types of anatomy to treat I think are well defined, we’re much better at both distal and proximal embolic protection works. We also know that still densely calcified lesions aren’t perfect, transversing the arch isn’t perfect, and what we talked about you heard from Dr. Metzger and Dr. Siddiqui, we know that we can have post dilatation to get the artery open but that’s the time of most embolization and that plaque starts to protrude through the stent but that embolic risk continues as Chris showed you, for some time afterwards. We know that it leads to both silent brain lesions by MRI that are higher than carotid endarterectomy, and the problem is that up until about a couple years ago in the United States, there’s been no real development.
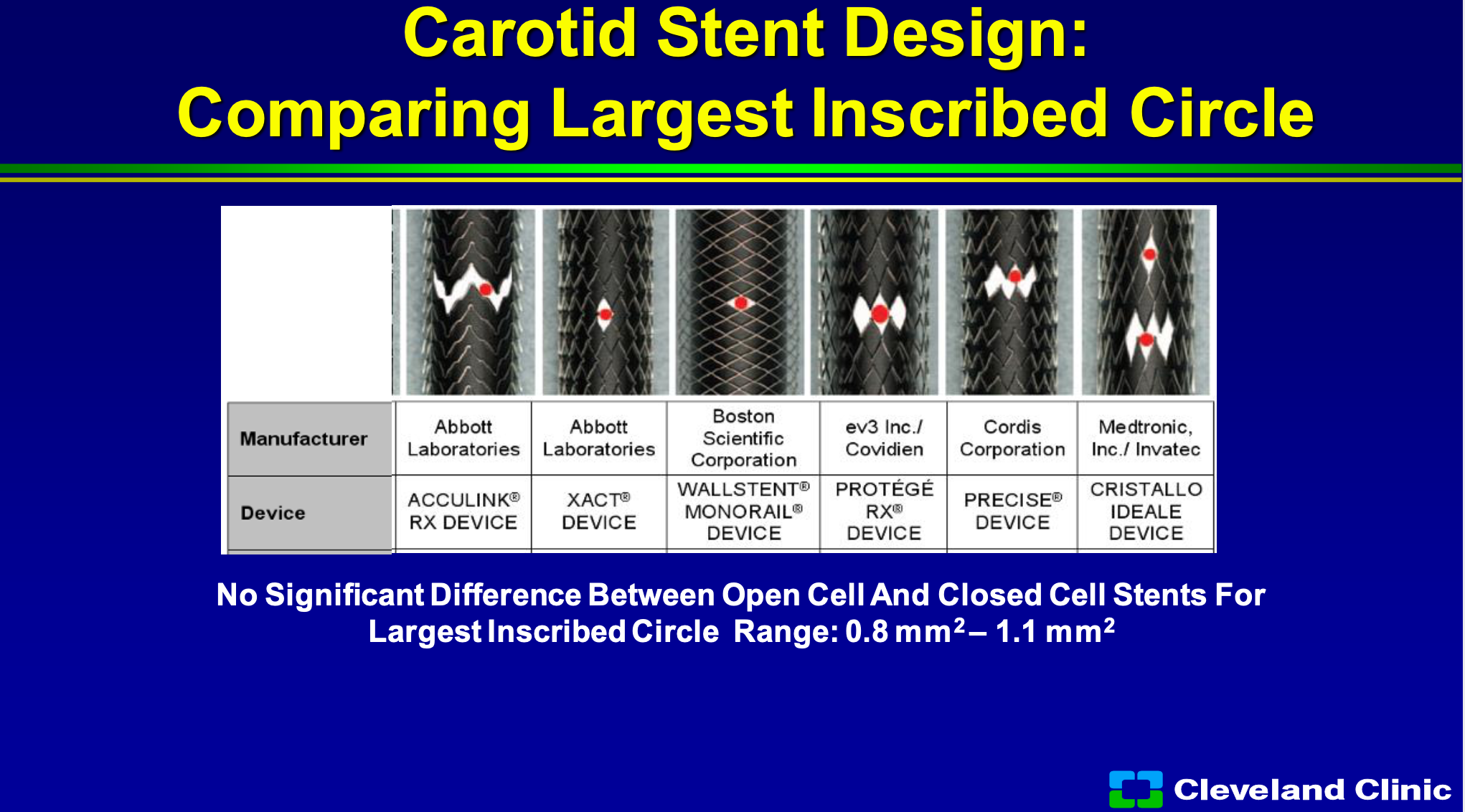
So if we look at some of the approved devices, whether open cell or closed cell, and you look at the area for a piece of plaque to go through, you can see that if you were a circle plaque they’re all about the same, but that’s in the straight artery, but if you start having a bending artery for an open cell design that plaque starts have a bigger area to go through. There’s not a big difference in the large circles between the closed cells to the open cells, but if you start having some tortuosity they can make a difference.

So what’s got me excited? It’s the micromesh net technology. There’s been three versions that have come out, but they’re not all the same. Some of them elongate and then have to foreshorten when you place them and don’t necessarily appose the plaque really well, some of them have had higher thrombosis risk, and I’m not going to go through the same data that either Marvin showed you, or Chris or Adnan showed you, but this micromesh from the CGuard from InspireMD is the largest amount of data set out there.
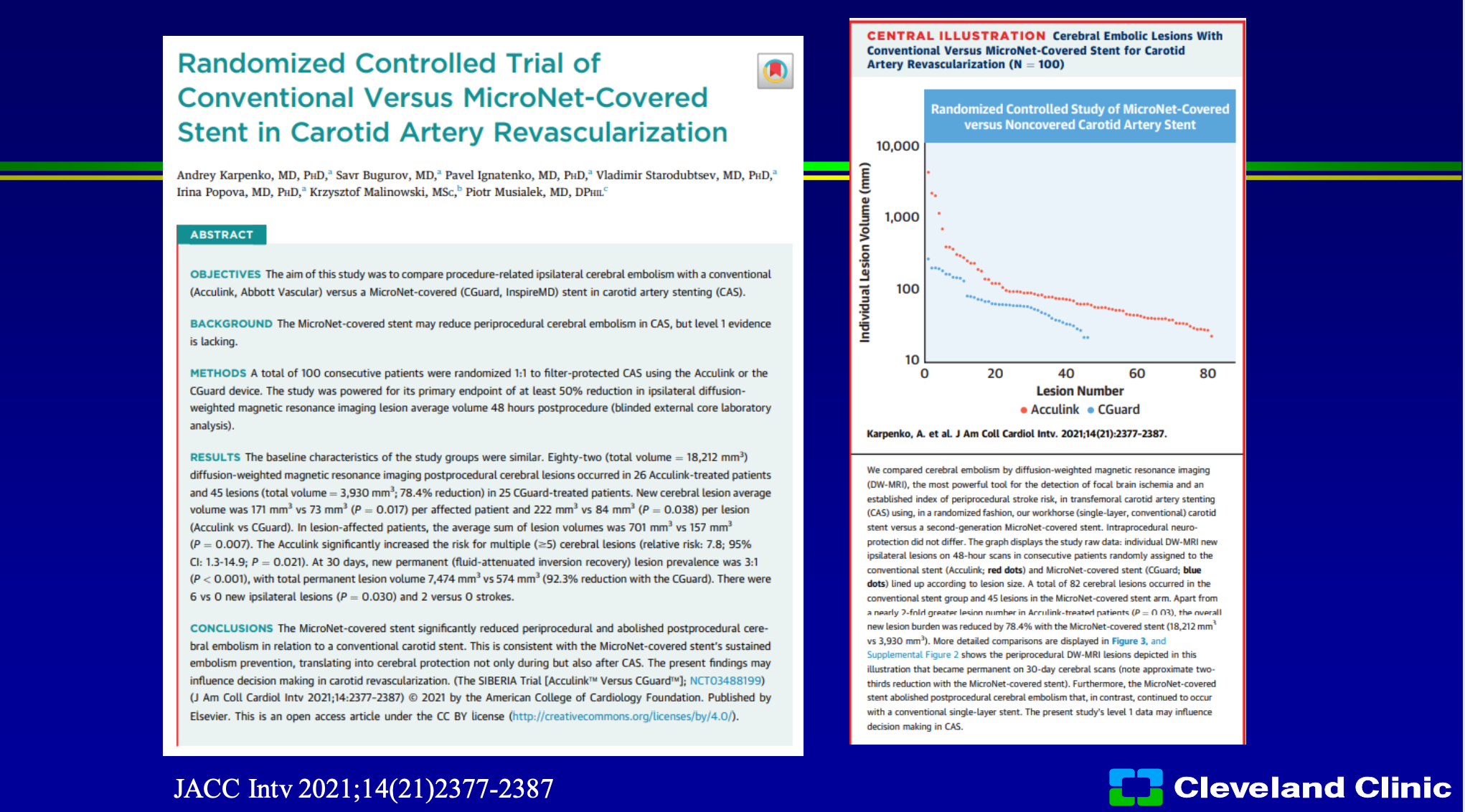
So we’ve heard that from CREST that surgery equivalent to stenting, but amazingly they’ve even spent time trying to study the CGuard stent against the Acculink stent which was the stent used in the CREST trial. So if you look here when people had CGuard or Acculink in a randomized study, there was much less event rate in it, and so not only do you say well we have a registry comparing one to the other, this was actually trial looking into two this was just published last year in JACC Interventions. I think this is one of those studies that really got me excited, and I think this is where this company is very well positioned because hopefully we’ll have this stent approved the United States, soon. Chris is the national PI, both Adnan and I are sites, I’ve used this clinically it works really well. I’ve seen the data from Europe was very strong and powerful but the advantage to me of the micromesh stent, and this design in particular, it conforms the vessel because it’s basically an open cell stent design, it’s going to stop plaque protrusion not only at the time of post dilatation, but outside of that because of how tight the weave is.
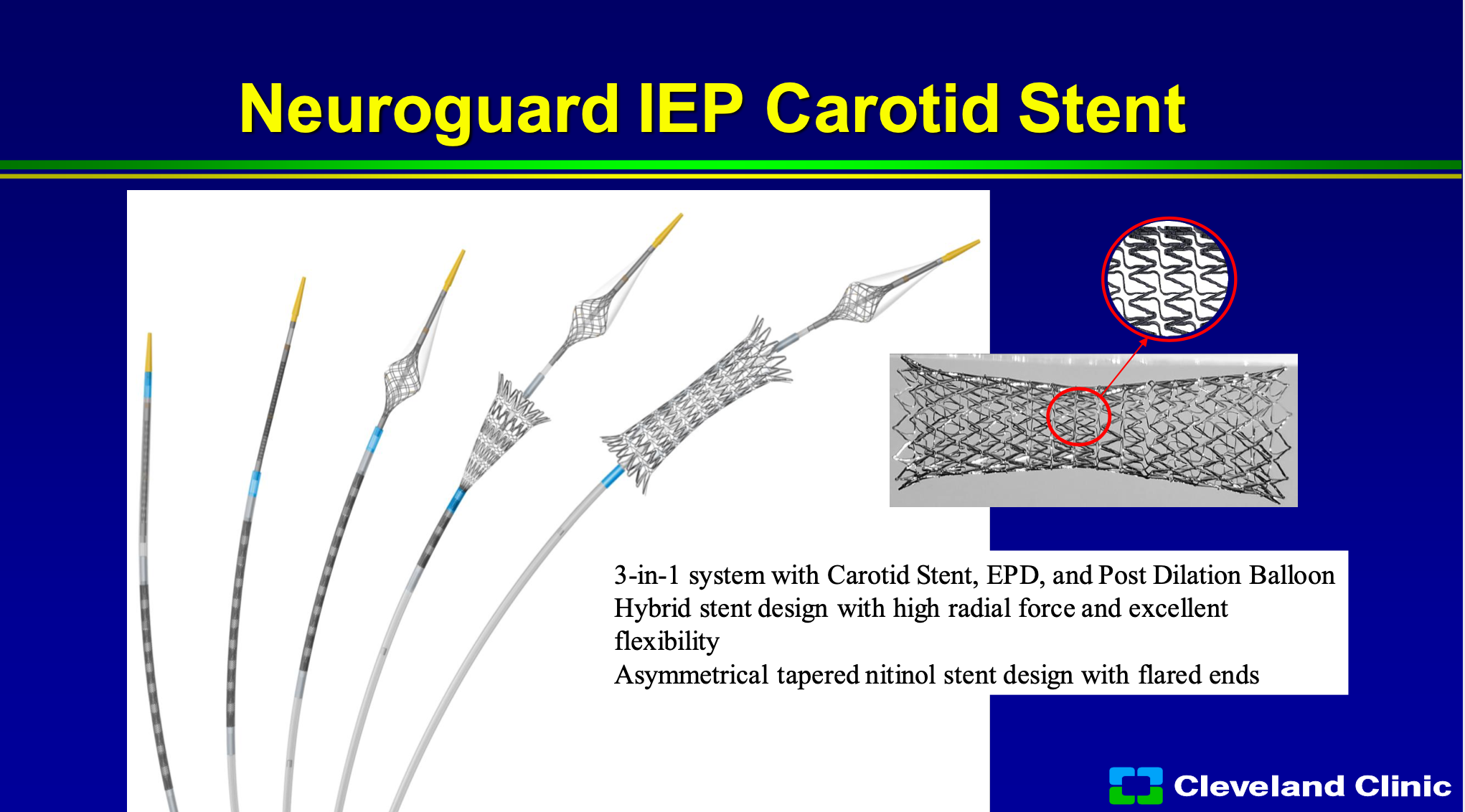
There are other technologies out there, one is the Neurogard protection device, and so that puts a second filter in and then you deploy the stent and you can post-dilate the stent. That will help protect any emboli while your ballooning and placing your stent, but as Chris showed a lot of those events happen later than that, so although this is a new technology, hopefully you address some of those needs. I don’t think it’s going to have the impact that the micromesh stents do because it’s only going to help stop issues right at the time of stent deployment and post dilatation.
So where are we at? I think carotid endarterectomy and carotid stenting, I agree with Chris there’s a lot more enthusiasm for carotid stenting now, and I believe, and we’re pushing to get CMS to relook at the NCD to cover for both greater than 50% symptomatic and greater than 70% asymptomatic patients just like they do for carotid endarterectomy. I think we’ve really spent a lot of time defining who best to use carotid stent technology over the last 20 years. TCAR is a great approach, it does have a role. I’m not a huge TCAR enthusiast but a lot of my vascular surgery friends use it almost more than surgery, and so the best part is this stent technology can be used there too. We’ve clearly seen now in the last year with this company advances in stent protection strategies and I think that’s going to continue to improve our outcomes, and so we definitely now know that the micromesh, in some versions, are better than the standard. I could say the three of us are all excited to hopefully have this in our hands to use as we want, how we want, in the United States soon, thank you.



