Why Invest in InspireMD
Our mission is clear: prevent stroke, save lives, and give physicians and patients the confidence to choose stenting—without compromise.
For over 15 years, InspireMD has been at the forefront of transforming vascular intervention. Our innovations in carotid stenting are designed to shift the market away from the limitations of open surgery and first-generation stents toward a platform that delivers best-in-class outcomes—the CGuard stent has been proven with more than 65,000 devices sold to date.
Our FDA-approved flagship product, CGuard® Prime, is the first and only mesh-covered carotid stent. It’s engineered to reduce peri-procedural stroke risk with unmatched clinical outcomes.
Transformation requires more than innovation—it demands strong reimbursement, supported outcomes, a world-class commercial and operational engine, and a capital structure built to fuel growth and establish a new standard of care.
We are poised and ready to execute.
CGuard Prime: Proven with Global Reach
CGuard Prime is powered by MicroNet™ mesh, our proprietary dual-layer technology that prevents plaque prolapse while preserving healthy blood flow.
Used in over 65,000 procedures worldwide, CGuard Prime demonstrated the lowest 30-day and 1-year primary endpoint major adverse event rates of any pivotal study in carotid intervention—and it’s now available in the U.S. market.
Key Data from the C-GUARDIANS trial
- 0.95% DSMI through 30 days
- 1.93% DSMI through 30 days plus ipsilateral stroke through 1 year1†
- 1% target lesion revascularization at 1 year
Positioned for Scalable Growth
With FDA approval and growing demand for safer, more effective solutions, InspireMD is uniquely positioned in the carotid and neurovascular markets. We've assembled top industry professionals, bringing immediate commercial access, deep clinical relationships, and operational excellence to fuel our next phase of growth. We’ve purposefully built a platform with an implant-first approach that supports all methods of carotid revascularization—including both CAS and TCAR—and is tailored to the multidisciplinary physician base performing these procedures.
Robust and consistently positive body of evidence from clinical trials
Low rates of major adverse cardiovascular events demonstrated in the C-GUARDIANS U.S. Investigational Device Exemption (IDE) trial
CGuard Prime demonstrated the lowest 30-day and 1-year primary endpoint major adverse event rates of any pivotal study of carotid intervention
Real-world, investigator-initiated studies position CGuard Prime as a potential standard of care in treating carotid artery disease
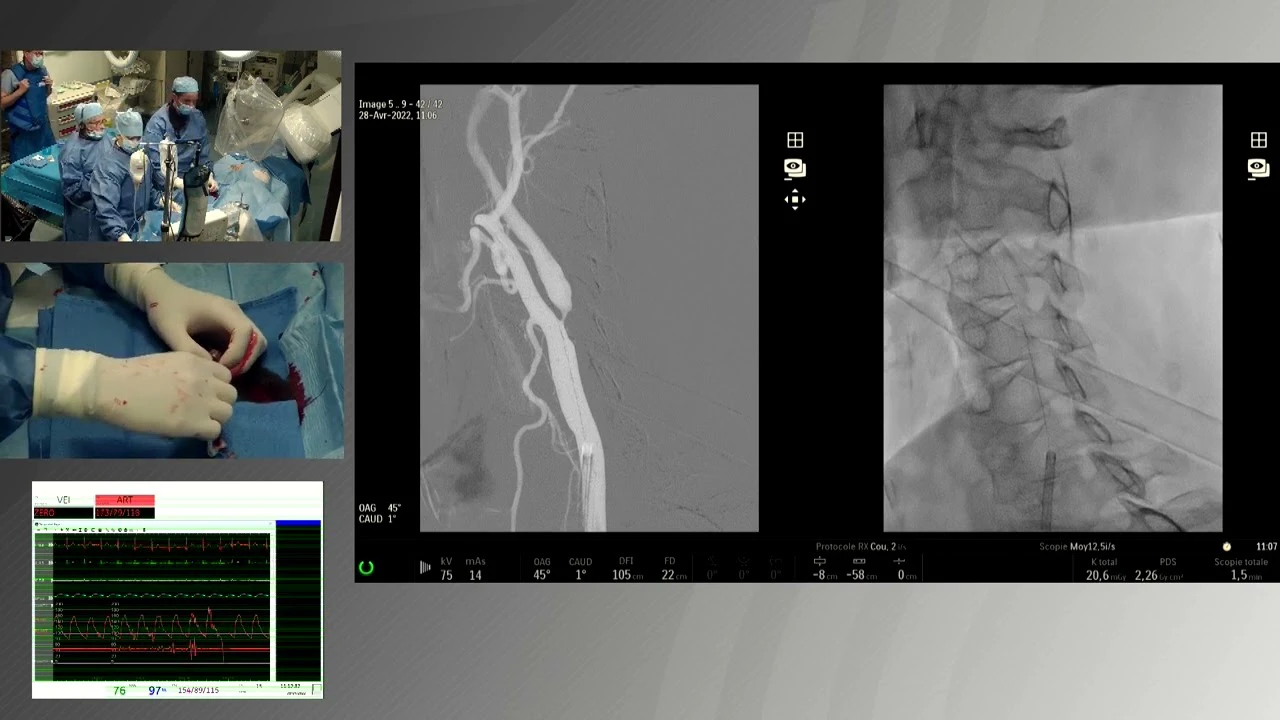
Severe Carotid Plaque Treated with CGuard™ EPS, Prof. Cremonesi
Ulcerated Carotid Stenosis treated with CGuard™, Prof. Musialek
Growth driven by innovative commercialization and dynamic expansion strategy
Serving 30+ countries*
- † Based on modified intent-to-treat Kaplan-Meier analysis
- 1. InspireMD, Inc. C-GUARDIANS Pivotal Trial: Primary Endpoint Clinical Study Report. Version F (Protocol PRO-9017).
- 2. ANVISA = the Brazilian Health Regulatory Agency
- * Through distributor channel partners with plans to go direct in the US.
Events

Webcast Registration for the Needham 24th Annual Virtual Healthcare Conference